Abstract
BACKGROUND AND PURPOSE: Granulomatous hypophysitis is a rare inflammatory condition of the pituitary gland with an imaging appearance that can overlap with that of pituitary adenoma. Differentiating the two before surgical resection can have important treatment implications. The purpose of our study was to determine whether it was possible to differentiate between granulomatous hypophysitis and pituitary adenoma on the basis of diffuse enhancing infrasellar basisphenoid bone marrow.
MATERIALS AND METHODS: We present 3 cases, initially thought to be pituitary adenomas, that were pathology-proved granulomatous hypophysitis. The preoperative MR images were reviewed for diffuse, enhancing infrasellar basisphenoid bone marrow. For comparison, we reviewed 100 cases of pathology-proved pituitary adenoma for the same finding. Additionally, imaging findings including the sphenoid sinus pneumatization pattern, clinical history, laboratory values, and pathology results were reviewed.
RESULTS: All 3 cases of granulomatous hypophysitis had diffuse enhancing infrasellar basisphenoid bone marrow. Conversely, this was not seen in any of the 100 pituitary adenomas. The patients with granulomatous hypophysitis were all women. Two patients had idiopathic granulomatous hypophysitis, and 1 had secondary granulomatous hypophysitis with sarcoidosis. Of the 100 patients with pituitary adenomas, 67 were women. The basisphenoid pneumatization patterns was as follows: 15 (type 2), 40 (type 3), and 45 (type 4).
CONCLUSIONS: We present 3 cases of granulomatous hypophysitis with diffuse enhancement of the infrasellar basisphenoid bone marrow that was not seen in our 100 cases of pituitary adenomas. This imaging feature may be valuable for suggesting a diagnosis of granulomatous hypophysitis and avoiding surgical resection of what might otherwise be misdiagnosed as a pituitary adenoma.
ABBREVIATIONS:
- AH
- autoimmune hypophysitis
- GH
- granulomatous hypophysitis
- LH
- lymphocytic hypophysitis
Granulomatous hypophysitis (GH) is a rare, inflammatory condition of the pituitary gland characterized by the presence and formation of granulomas throughout or around the pituitary gland. It is most often associated with systemic diseases such as sarcoidosis or tuberculosis (secondary GH) and, less commonly, isolated to the gland (idiopathic GH). GH forms a subset of autoimmune hypophysitis (AH), which also includes lymphocytic hypophysitis (LH) and Langerhans cell histiocytosis in the differential.1,2
The imaging appearance of AH is nonspecific and overlaps with that of pituitary adenomas, with 1 prior study describing a grading scale to distinguish these entities.3 In terms of GH, there are scant case reports on the imaging findings, and they also overlap with those of pituitary adenomas.4-10 The purpose of this study was to test our observation that diffuse enhancement within the basisphenoid marrow below the sella can distinguish GH from a pituitary adenoma, a feature that is important diagnostically because medical and surgical management differs for these conditions.11,12
MATERIALS AND METHODS
We retrospectively reviewed an internal database for pathology-proved GH with preoperative contrast-enhanced MR imaging with a small FOV focused on the sella. T2-weighted, precontrast T1-weighted, and postcontrast T1-weighted images were reviewed for edema and enhancement of the basisphenoid bone marrow. Additionally, the patients’ electronic medical records were reviewed for clinical history, surgical pathology, and relevant laboratory results.
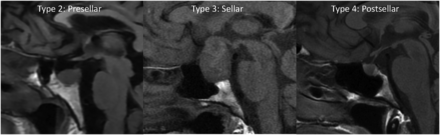
Our internal database was also reviewed for 100 consecutive pathology-proved pituitary adenomas with preoperative MR imaging from January 2021 to January 2022. Images were primarily reviewed for edema and enhancement of the basisphenoid bone marrow. Additionally, the pneumatization pattern of the sphenoid sinus can limit bone marrow evaluation; therefore, the sphenoid sinus pneumatization was evaluated and graded as follows (Fig 1): type 1, conchal; type 2, presellar; type 3, sellar; and type 4, postsellar.13 For the adenoma cases, we measured the maximum diameter and recorded their prolactin levels.
Precontrast T1-weighted sagittal images show the 3 types of sphenoid sinus pneumatization seen in our patients. Type 2 (presellar): the posterior wall of the sphenoid sinus is in front of the anterior wall of the sella turcica; type 3 (sellar): the posterior wall of the sphenoid sinus is between the anterior and posterior wall of the sella turcica; type 4 (postsellar): the posterior wall of the sphenoid sinus is located behind the posterior wall of the sella turcica. An example of type 1 (conchal type, minimal air in the sphenoid sinus) is not shown because this is uncommon and we did not have a patient with this pneumatization pattern.
All cases were reviewed by a neuroradiology instructor (I.T.M.). Cases with equivocal findings were additionally reviewed by a neuroradiologist with 20 years of experience (C.M.G.). Basic statistical analysis was performed with Excel (Microsoft).
RESULTS
Patients with GH
Three cases of pathology-proved GH are presented below. Each patient had bone marrow enhancement of the basisphenoid below the sella.
Patient 1.
A 51-year-old woman presented to an outside institution with headache, dizziness, hyponatremia (113 mEq/L), and emesis with 11 kg of unintentional weight loss (Fig 2). Her laboratory values were consistent with central hypothyroidism, and she was treated with levothyroxine. The contrast-enhanced MRI showed a 1.8-cm sellar mass with type 3 pneumatization of the sphenoid sinus and enhancing bone marrow edema of the basisphenoid. The diffuse enhancement spanned nearly the entire anterior-posterior and transverse dimensions of the bone marrow beneath the sella. She was thought to have a pituitary macroadenoma and underwent endoscopic transsphenoidal resection of the central pituitary gland. The pathology demonstrated GH. Staining for acid-fast Bacillus was negative. She did not have a history of systemic granulomatous disease, and her pituitary lesion was thought to represent primary, idiopathic GH.
MR images of patient 1 with idiopathic GH and type 3 (sellar) sphenoid sinus pneumatization. Precontrast T1-weighted sagittal image (A) shows low signal in the infrasellar basisphenoid bone marrow. This low signal corresponds to bone marrow enhancement below the sella (arrows) seen on sagittal (B) and coronal (C) T1-weighted fat-saturated postcontrast images.
Patient 2.
A 30-year-old woman presented with right orbital headaches, polydipsia, and polyuria (Fig 3). She was found to have hyperprolactinemia (49.5 μg/L) and a 1.7-cm sellar mass with type 3 pneumatization of the sphenoid sinus and diffuse enhancing bone marrow of the basisphenoid below the sella on MRI, thought to be a pituitary adenoma. She underwent a pituitary biopsy, findings of which were consistent with GH. Following the biopsy, she was treated with dexamethasone and desmopressin. Follow-up MRI 6 months later showed a decrease in the size of the pituitary gland and resolved bone marrow enhancement of the basisphenoid. A cervical lymph node biopsy for work-up of sarcoidosis was negative. She did not have a diagnosis of a granulomatous systemic disease, and her pituitary lesion was thought to represent primary, idiopathic GH. At follow-up 1.5 years after biopsy, the patient did not have symptoms of hypopituitarism, and her diabetes insipidus had resolved.
MR images of patient 2 with idiopathic GH and type 3 (sellar) sphenoid sinus pneumatization. This is a collage of images of a single patient at baseline before intervention (upper row) followed by postbiopsy and post-steroid treatment. Left to right, Coronal T2-weighted, precontrast sagittal T1-weighted, fat-saturated postcontrast sagittal T1-weighted, and fat-saturated postcontrast coronal T1-weighted images show diffuse infrasellar basisphenoid bone marrow–enhancing edema (arrows) on baseline that resolved 6 months later after biopsy and steroids.
Patient 3.
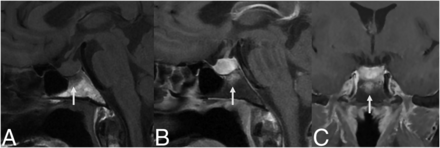
A 34-year-old woman presented with 3 months of progressive headache, gastrointestinal upset, amenorrhea, fatigue, and blurry vision (Fig 4). She was found to have hyperprolactinemia (78.1 ug/L) and a 1.6-cm sellar mass with type 3 pneumatization of the sphenoid sinus and diffuse enhancing edema of the basisphenoid below the sella, thought to be a pituitary adenoma. She underwent an endoscopic transsphenoidal exploration with a biopsy that revealed granulomatous inflammation. After treatment with oral dexamethasone, a follow-up MRI 7 months later showed a marked decrease in the size of the pituitary gland with resolved enhancing edema of the basisphenoid. One month after her pituitary biopsy, she had a stomach biopsy that was positive for non-necrotizing granulomas, and her pituitary lesion was thought to represent secondary GH in the setting of sarcoidosis.
MR images of patient 3 with secondary GH from sarcoidosis. These show preoperative edema (A, coronal T2-weighted image) and enhancement (B, postcontrast coronal T1-weighted image) of the infrasellar basisphenoid bone marrow. After biopsy and oral dexamethasone, follow-up imaging 7 months later shows resolution of the edema (C, coronal T2-weighted image) and enhancement (D, postcontrast coronal T1-weighted image) with a decreased size of the pituitary mass.
Pituitary Adenoma.
Of the 100 patients with pathology-proved pituitary adenomas, 67 were women and 33 were men. The average age was 53.3 (SD, 17.3) years (range, 19–84 years). The basisphenoid pneumatization patterns were as follows: 15 (type 2), 40 (type 3), and 45 (type 4). None of the 100 patients had diffuse basisphenoid enhancement (Fig 5). One patient had focal enhancement of the basisphenoid (Fig 6) but did not have the diffuse enhancement seen in the 3 GH cases. The adenomas had an average maximum diameter of 2.6 cm (range, 0.4–4.4 cm). The average prolactin level was 103.4 μg/L (range, 0.3–1781.0 μg/L); however, when we excluded 3 outlier patients with markedly high prolactin levels (1189, 1750, and 1781 μg/L), the average was 54.9 μg/L.
MR images of 2 patients with large pituitary adenomas demonstrate the typical nonenhancing basisphenoid bone marrow. Precontrast (1A/2A) and fat-saturated postcontrast (1B/2B), sagittal T1-weighted images without basisphenoid bone marrow enhancement. Both patients have type 2 pneumatization of the sella.
A large pituitary adenoma on precontrast sagittal T1-weighted (A), postcontrast sagittal (B), and coronal (C) T1-weighted images shows focal bone marrow enhancement in the right anterior and lateral aspect of the basisphenoid. This is a distinct pattern from the diffuse enhancement in patients with GH.
DISCUSSION
Our study presents 3 cases of pituitary GH with diffuse basisphenoid bone marrow enhancement that was not found in any of our cases of pituitary adenoma. Differentiating pituitary adenoma and GH can have a large impact on patient care. Pituitary adenomas, as in all 100 of our cases, are frequently resected. GH, on the other hand, can be treated with steroids after diagnosis with biopsy and can potentially spare the pituitary function.12 We found that the diffuse enhancement of the basisphenoid below the sella can be used to distinguish GH from pituitary adenoma.
GH can be seen primarily in the sella or found in association with systemic disease such as sarcoidosis and tuberculosis. Histologically, GH is an inflammatory process with granulomas and multinucleated giant cells.3 Therefore, it should not be surprising that the adjacent bone of the basisphenoid below the sella had enhancement in the 3 cases that we present. However, this has not been described with LH, which, along with GH, is considered a subtype of AH. The relationship between LH and GH is controversial, with some believing that they represent opposite ends of the spectrum, while others report that they are 2 separate diseases.11 Unlike LH, which has a greater affinity for women and typically involves the late pregnancy or early postpartum periods, GH does not preferentially affect women or have an association with pregnancy.3
The literature contains several case reports of GH, but they largely describe nonspecific imaging findings and do not assist in differentiation from adenomas.4-10,14 In a systematic review, Hunn et al12 examined the MR imaging findings of 51 cases of GH. Their most frequent MR imaging finding was isointense T1 lesion signal in 29.4% of patients. The next most frequent finding was loss of the posterior pituitary bright spot (19.6%), followed by T2-hyperintense signal (15.7%).
Vasile et al15 presented a case of GH and summarized 7 cases from the literature with MR imaging findings that were nonspecific: T1 isointense to brain (4/7 cases), heterogeneous T2 signal (2/2 cases), and homogeneous enhancement (3/7 cases). This work did state that findings of inflammation including dural enhancement, sphenoid sinus mucosal thickening, and bone marrow abnormality could be seen; however, abnormal bone marrow was not further explained. Bhansali et al6 described pituitary stalk thickening and loss of the posterior pituitary bright spot as clues to the diagnosis of GH, but again, these are nonspecific to this diagnosis.
Gutenberg et al3 proposed a scoring system to distinguish AH and pituitary adenoma on the basis of an evaluation of 19 clinical and imaging features. However, their study examined all patients with AH, combining a smaller number of patients with GH and a larger number of those with LH. The case-control study included only 46 biopsy-proved cases of GH of 402 patients (11.4%). The most useful clinical feature to distinguish AH and pituitary adenoma was pregnancy; however, this is a key feature that differentiates LH and GH. Unfortunately, enhancing edema of the basisphenoid was not studied. One of their MR imaging features highly indicative of AH was loss of the posterior pituitary bright spot; however, 30% of their large adenomas had the same finding. Similarly, Saeki et al16 reported that the posterior pituitary bright spot was nonvisible in 20% of large adenomas, which can also be seen in nonpathologic conditions such as dehydration.17
A case report by Kartal et al18 described clival enhancement in a patient with lymphocytic hypophysitis. However, on their fat-saturated postcontrast figure, the enhancement was along the dorsal cortex of the clivus and could very well represent dural thickening and/or venous congestion. In fact, their images clearly demonstrated no basisphenoid bone marrow enhancement. Nakata et al19 described T2 parasellar dark signal in cases of LH, which we did not see in our cases of GH.
Our study has several limitations, the first being the small sample size. GH is a rare disease and does not necessitate a biopsy if there is known systemic disease; therefore, we were only able to present 3 pathology-proved cases. However, all cases had stark diffuse enhancement of the basisphenoid bone marrow below the sella, which is distinctly different from findings in pituitary adenomas. Additionally, the identification of enhancing bone marrow depends on having bone marrow to evaluate; therefore, type 4 pneumatization patterns are difficult to evaluate in the bone marrow and comprised 45% of our pituitary adenoma cases. Additionally, if the postcontrast images are not fat-saturated, evaluating true enhancement from the T1-hyperintense signal of fatty bone marrow would be extremely limited. While GH is a rare disease, the implication for patient treatment compared with pituitary adenoma suggests that preoperative pituitary MR imaging protocols should contain postcontrast images with fat saturation. While basisphenoid bone marrow enhancement is not necessarily specific to GH, we could not find studies describing this in other inflammatory pathologies (abscess, LH), and more important, we did not find it any of our cases of pituitary adenoma.
We did not evaluate other pathologies such as Rathke cleft cysts, intrasellar craniopharyngioma, or metastases. Finally, cases of infection, including sphenoid sinusitis and skull base osteomyelitis, could lead to bone marrow edema; however, clinical history as well as the epicenter of bone marrow edema could help to differentiate these cases, because GH primarily involves the bone marrow directly beneath the sella.
CONCLUSIONS
We present 3 cases of GH with diffuse enhancement of the infrasellar basisphenoid bone marrow that was not seen in our review of 100 cases of pituitary adenomas. This imaging feature may be valuable to suggest a diagnosis of GH and avoid resection of what would otherwise be mistaken for a pituitary adenoma.
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received March 25, 2022.
- Accepted after revision June 28, 2022.
- © 2022 by American Journal of Neuroradiology