Abstract
SUMMARY: Intraosseous venous malformations represent a subtype of venous vascular malformations that arise primarily in bone. In the head and neck, intraosseous venous malformations are most frequently found in the skull, skull base, and facial skeleton, with location at the geniculate ganglion of the facial nerve perhaps the most widely recognized. These non-neoplastic lesions are characterized by dilated venous channels with characteristic internal bony spicules on CT but may present with a more complex appearance on MR imaging and may share features with more aggressive lesions. Further confounding the imaging-based diagnosis of intraosseous venous malformation is the frequent misrepresentation of these lesions as hemangiomas in the radiology and clinical literature, as well as in daily practice. Because most intraosseous venous malformations can be left alone, their correct diagnosis may spare a patient unnecessary concern and intervention.
ABBREVIATIONS:
- FN
- facial nerve
- FS
- fat saturated
- GLUT1
- glucose transporter 1
- IOVM
- intraosseous venous malformation
- ISSVA
- International Society for the Study of Vascular Anomalies
Venous malformations are part of the spectrum of vascular malformations designated by their vessel of origin, based on the widely accepted classification of vascular anomalies endorsed by the International Society for the Study of Vascular Anomalies (ISSVA).1 They represent congenitally malformed and dilated venous channels with sluggish internal flow that occur commonly in soft tissue; in the head and neck, the muscles of mastication are a particularly frequent site. Intraosseous venous malformations (IOVMs) are less common compared with soft-tissue venous malformations in the head and neck. When found within the facial skeleton, calvaria, and skull base, IOVMs may demonstrate an aggressive appearance, with medullary space expansion, enhancement, and cortical thinning. Identification of coarsened internal trabeculae, best appreciated on CT, is nearly pathognomonic of this entity2,3 and may obviate the need for biopsy.
Incorrect nomenclature is often applied to soft-tissue vascular malformations within the head and neck. This is particularly true of cavernous venous vascular malformations within the orbit, cavernous sinus, or brain parenchyma, which consist of cavernous venous spaces lined by endothelial cells. Because these lesions have a pseudoencapusulated appearance, they are often inappropriately referred to as “cavernous hemangioma” or “angioma,” suggesting neoplasia rather than the more appropriate designation of “cavernous venous malformation.”
Similarly, a major factor in the confusion surrounding the correct diagnosis of vascular malformations within bone derives from the inconsistency and inaccuracy in nomenclature used in the small body of literature on the topic. These lesions are frequently incorrectly referred to as “ossifying hemangiomas” or when in the temporal bone, as “facial nerve hemangioma” and qualified as “cavernous” or “capillary.” The more apt description of such lesions as venous malformation was first introduced by Mulliken and Glowacki,4 in 1982, who proposed a classification scheme based on the pathophysiology of vascular lesions and differentiated between vascular tumors and vascular malformations. In this binary classification system, the term “hemangioma” is reserved for the common benign vascular tumor of infancy, a true neoplasm that grows by endothelial hyperplasia and expresses markers of cellular proliferation such as vascular endothelial growth factor and cell nuclear antigen, as well as the immunohistochemical marker glucose transporter protein-1 (glucose transporter 1 [GLUT1]). Hemangiomas additionally manifest a unique triphasic growth pattern, with rapid enlargement during infancy followed by spontaneous involution and, typically, complete resolution by mid-childhood. Conversely, vascular malformations are non-neoplastic, static errors of vascular morphogenesis that will not regress spontaneously and are composed of dysmorphic forms of the vessels of origin (capillary, venous, lymphatic, arterial) with growth occurring on the basis of progressive vascular ectasia, rather than cellular proliferation. IOVMs are part of the latter category, representing dilated and ectatic venous channels within bone. In fact, it has been argued that true intraosseous hemangiomas do not exist, and one should assume that all such lesions identified within bone represent IOVM.5 This is true not only for venous malformations of the head and neck but for those found in vertebral bodies as well.6 In 2014, the ISSVA published an updated classification based on the work of Mulliken and Glowacki,4 similarly classifying lesions as vascular tumors and vascular malformations.7
Although the nomenclature of Mulliken and Glowacki4 has been in existence for >30 years, incorrect terminology remains prevalent in the literature. Hassanein et al8 performed a literature review and found the term “hemangioma” incorrectly used in about 70% of all articles published in 2009; most important, 20% of these patients received inappropriate therapy because of this seemingly trivial error in nosology. Whereas infantile hemangioma causing substantial disturbance in form or function may be treated pharmacologically, venous malformations will not respond to antiangiogenic treatment. Sclerotherapy, possibly in combination with surgical excision, may be required.5 Fábián et al9 performed a literature search for terminology used for intraosseous vascular lesions, including intraosseous hemangioma and “intraosseous malformation” of the face. Of 272 cases reported between 1950 and 2016, 50.7% were incorrectly described as hemangiomas.9 Further analysis, however, demonstrated that before the first ISSVA publication in 1996, almost 91% of cases were reported as intraosseous hemangiomas, while in subsequent years, usage of this incorrect terminology dropped to 39%.9
Despite increasing familiarity with the ISSVA classification and nomenclature on the part of clinicians and researchers, much work remains regarding the use of correct terminology when dealing with vascular lesions. It is the authors’ opinion that the incorrect nomenclature should not be used in reports, even if discrepant from the vernacular used by referring physicians, because inappropriate descriptions of a vascular malformation such as a hemangioma will only further serve to perpetuate confusion surrounding the topic. Instead, the authors suggest using the correct term and then adding “previously known as hemangioma” within the report. Clarification and collegial education regarding the choice of terminology might accompany the report via phone or email communication to the ordering provider. Table 1 shows commonly used terms and the corresponding correct terminology.
Commonly used terms and the corresponding correct terminology
Like all vascular malformations, IOVMs are present at birth and will grow in tandem with the somatic growth of the child, occasionally punctuated by periods of more rapid enlargement stimulated by hormonal, inflammatory, or traumatic factors.10 Politi et al11 report a female predilection, which suggests possible endothelial growth by hormone stimulation. Similarly, malformations may present in adolescence during growth spurts and presumably also under the influence of endogenous hormones. Within the lesion, repetitive intralesional hemorrhage may contribute to lesion expansion. IOVMs are often discovered incidentally on imaging studies performed for an unrelated indication; though in more superficial locations, such as in the zygoma, they may be visible or palpable. In the temporal bone, IOVMs are most frequently identified adjacent to the geniculate ganglion of the facial nerve. At this site, clinical presentation may be of progressive facial nerve paresis. Given that most lesions are asymptomatic, the overall prevalence of IOVMs in the population is difficult to estimate, though they are thought to represent <1% of osseous neoplastic and tumorlike lesions.12 Treatment is predicated on symptoms; incidentally discovered, asymptomatic lesions do not require therapy. IOVMs that result in cosmetic or functional issues are treated by surgical excision.13,14
On gross pathology, IOVMs are described as spongy or porous, raised hemorrhagic lesions, often with ill-defined margins relative to adjacent normal bone. On histopathologic analysis, mature bony trabeculae are separated by thin-walled dilated cavernous vascular spaces that are lined by flattened endothelial cells.15⇓-17 Immunohistochemical analysis can definitively distinguish IOVMs and other vascular malformations from infantile hemangiomas by assaying for GLUT1.10,18
IOVMs demonstrate low-to-intermediate signal intensity on T1WI; there may be areas of sporadic internal T1-shortening at sites of hemorrhage or thrombosis.19 On T2WI, IOVMs will demonstrate heterogeneous, high signal intensity.2 There is heterogenous, avid contrast enhancement, which may be delayed.20 Internal areas of signal void may be visible, often in a spiculated “sunburst” pattern, representing the characteristic radiating internal bony trabeculae. The sunburst pattern results from bony displacement by a network of vascular spaces, with reactive new bone formation resulting in thickened trabeculae. When these trabeculae are viewed in cross-section, a “honeycomb” or “soap bubble” appearance is described. On CT, this trabecular pattern will be highlighted against a background of an expansile lucent osseous lesion, with intact cortical margins. Phleboliths, appearing as rounded areas of signal void on MR imaging and calcification on CT, are occasionally identified, though they are considerably less frequent in IOVMs compared with their soft-tissue counterparts. Because IOVMs are low-flow lesions, there is no role for either cross-sectional or catheter angiographic studies in their diagnosis or characterization. IOVMs may demonstrate an atypical appearance on MR imaging when relatively lipid-poor and may also exhibit a more aggressive-type behavior, leading to soft-tissue extension, cortical expansion, and potentially pathologic fracture.21⇓-23 Some authors postulate that aggressive features are more commonly seen in underlying combined capillary-venous malformations, particularly in the familial cerebral cavernous malformation population.22 The presence of other features such as homogeneous soft-tissue enhancement, honeycomb pattern of internal trabeculation, T1 hyperintensity, absence of periosteal reaction and lack of erosive change, help to support the correct diagnosis despite otherwise atypical features.2,24⇓⇓-27 Such lesions can still be referred to as IOVMs. Differential diagnoses for less characteristic appearances will vary by site but may include intraosseous meningioma, fibrous dysplasia, osteochondroma, osteosarcoma, and ossifying fibroma.19
Subsites of IOVM in the Head and Neck: Calvarium
First described in 1845 by Toynbee and perpetually miscategorized as cavernous or osseous hemangiomas, calvarial IOVMs present clinically as slowly growing or indolent firm, nonmobile masses with freely mobile overlying skin.11,28 They are often encountered on CT or MR imaging of the brain as incidental findings and may prove problematic if misdiagnosed. Because the lesion is composed of malformed venous channels within bone trabeculae, calvarial IOVMs primarily occur within the diploic space with an expansile appearance and thinning of the overlying cortex. This appearance is best delineated with high-resolution CT imaging, which easily depicts the characteristic elements, variously described as stippled, spiculated, honeycomb, spoke wheel, or sunburst in appearance (Figs 1 and 2).20 The variable ossific density is thought reflective of osteoblastic activity from chronic and repeat hemorrhage.28
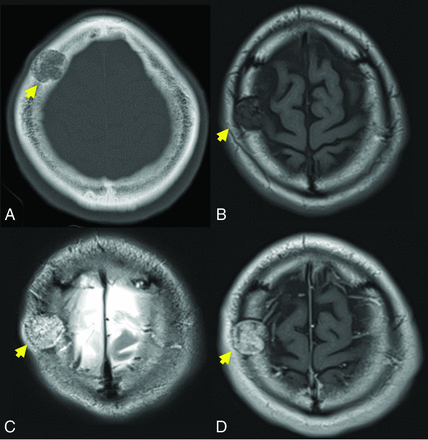
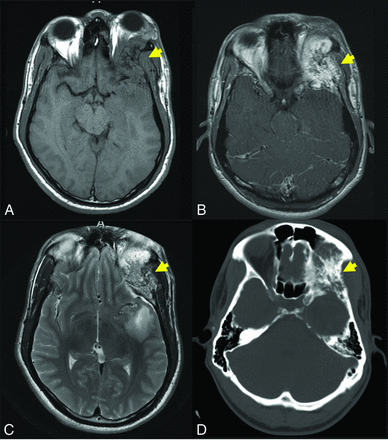
A 39-year-old man with a slowly growing calvarial mass. Axial CT (A) demonstrates a lucent right frontal bone lesion centered in the diploic space with a honeycomb pattern of internal calcification (arrow). Note a minimal overlying scalp deformity; the patient was able to palpate this slowly growing lesion. The lesion (arrow in B–D) demonstrates intermediate signal on T1WI (B), marked hyperintensity on T2WI (C), and prominent contrast enhancement, T1WI C+ (D). Note the internal foci of low signal most prominent on the T2-weighted examination (C) corresponding to the internal bone spicules evident on the CT. The diagnosis of IOVM was made on the basis of the characteristic imaging appearance. C+ indicates with contrast.
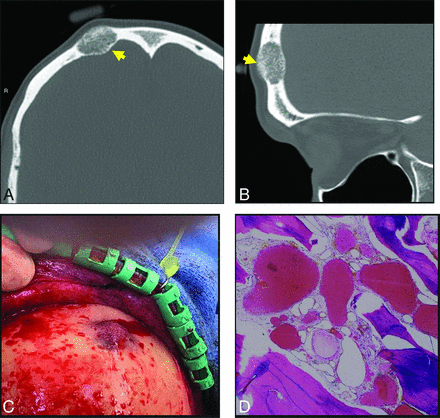
A 40-year-old man with a palpable frontal bump. Axial (A) and sagittal (B) thin-section CT images demonstrate a typical expansile lucent lesion with internal spiculated calcification (yellow arrow, A and B). Intraoperative photograph (C) shows a raised, red, porous-appearing calvarial lesion. H&E-stained photomicograph (D) shows dilated cavernous blood-filled spaces lined by flattened epithelium, characteristic of a venous malformation. There is focal hemosiderin staining, which is evidence of prior hemorrhage.
The imaging appearance of calvarial IOVMs with MR imaging is variable. IOVMs have strikingly hyperintense signal on T2-weighted imaging, reflecting slow-flowing blood or subacute thrombus. On T1-weighted imaging, some lesions have high signal from thrombus or fat, which may be differentiated with a fat-suppressed technique. Residual cortex has thin and hypointense signal on all MR pulse sequences, and the internal ossific spicules may be evident as internal areas of signal void (Fig 1C). Extraosseous soft-tissue extension is possible and better discriminated on MR imaging, given its advantage in soft-tissue contrast resolution.
The differential diagnosis includes intraosseous meningioma,29,30 also showing avid contrast enhancement and T2 hyperintensity,31 though typically presenting in an older age group and with associated osseous sclerosis. Fibrous dysplasia on CT classically has a ground-glass or heterogeneously cystic appearance and intermediate-to-low T2 signal intensity reflecting its fibrous content.29 Dermoid and epidermoid cysts typically demonstrate low CT density without an internal matrix, without enhancement on MR imaging and with characteristic diffusion restriction. When a calvarial lesion is large or growing at a worrisome pace, malignant lesions such as metastasis or myeloma or inflammatory lesions such as eosinophilic granuloma may be considered, and prompt lesion excision and/or tissue sampling may be necessary.32 As with most lesions at the skull or skull base, diagnostic accuracy is improved when both the CT and MR imaging appearance are co-interpreted as complementary examinations. Table 2 summarizes typical CT and MR imaging findings of IOVM and other lesions within the imaging differential diagnosis.
Imaging findings of IOVM and common lesions in the differential diagnosis
Facial Skeleton
As expected, primary IOVMs of the facial bones are more likely than calvarial lesions to present clinically as a recognizable deformity and firm, palpable masses. The maxilla is most frequently involved, with classic locations for IOVM at the malar eminence, orbital rim, and zygomaticomaxillary junction (Figs 3 and 4). Less common sites include the nasal bones and mandible.33 On occasion, the facial bones may be secondarily involved with more complex and transspatial venous malformations of the facial soft tissues. Dentition may be altered as result of a maxillary or mandibular IOVM, though jaw deformity is more commonly encountered with arteriovenous malformations.34 Surgical treatment is often pursued to correct the cosmetic deformity and/or to restore function. Imaging is necessary not only for lesion characterization and anatomic mapping before surgery but also for providing image data for navigational surgical planning. In the setting of large IOVMs, preoperative lesion embolization may be offered.24
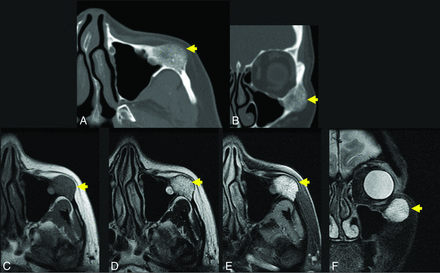
A 56-year-old woman with a palpable facial mass. The yellow arrow indicates a lesion in A–F. Axial (A) and coronal (B) thin-section CT in bone windows demonstrates an expansile, low-density lesion with internal coarsened trabeculae. The characteristic internal honeycomb pattern is typical of an IOVM. Axial T1 C– (C) and axial T1 C+ FS (D) sequences demonstrate a T1-intermediate, avidly enhancing lesion that extends beyond the cortical margin; the cortex is thinned but identifiable. Axial T2 FS (E) and coronal T2 FS (F) sequences demonstrate that the lesion is markedly T2 hyperintense. The diagnosis of IOVM was made on the basis of the characteristic imaging appearance, and the lesion remained stable on surveillance imaging for 3 years. C+ indicates with contrast; C–, without contrast.
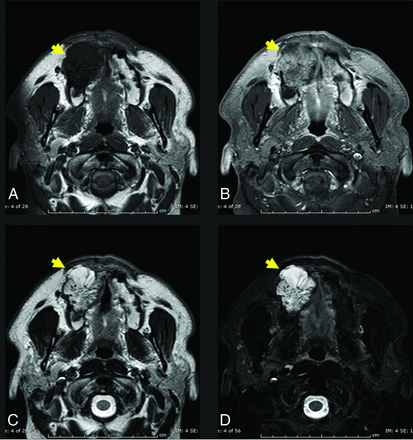
Axial T1 (A) and axial T1 C+ FS (B) images through the maxilla demonstrate a T1-intermediate, heterogeneously enhancing expansile lesion centered in the right maxillary alveolus (yellow arrow, A and B). The lesion extends beyond the cortical margin, and there is poor identification of the normal cortex. Axial T2 (C) and axial T2 FS (D) sequences demonstrate that the lesion is T2-hyperintense with internal T2-hypointense trabeculae (yellow arrows, C and D). The diagnosis of IOVM was confirmed on pathology. C+ indicates with contrast.
Differential diagnostic considerations for facial IOVMs are as previously described for calvarial IOVMs and include fibrous dysplasia, primary bone neoplasm, metastasis, and myeloma. Mandibular or maxillary osteosarcoma may result in a sunburst periosteal reaction, though this is typically limited to the periphery of the lesion.35 Of course, the rapid growth of osteosarcoma represents a substantially different clinical course than IOVM.
Skull Base
Given the deep location of skull base IOVMs, clinical signs and symptoms are rare, and these lesions are almost exclusively discovered incidentally at imaging for other indications. The skull base is a rarely reported site of IOVM, and it is often not considered as a leading differential diagnosis for an expansive bony mass in this location.19 Instead, it may be mistaken for malignancy or other osteoblastic or sclerotic lesions such as meningioma, Paget disease, or fibrous dysplasia. The greater sphenoid wing is a common location for an intraosseous meningioma, also known as an en plaque or hyperostotic meningioma.36 Both lesions may expand bone, but intraosseous meningiomas more often result in uniform bony sclerosis rather than lucency and do not typically demonstrate the trabecular matrix characteristic of IOVMs (Fig 5). The internal areas of signal void within a posterior skull base IOVM may simulate the “salt and pepper” MR imaging appearance of a glomus jugulare tumor; however, CT will readily distinguish these entities because paragangliomas lack an internal calcified matrix (Fig 6). Additionally, careful consideration of anatomy will localize these lesions to the bone rather than skull base foramina. Within the central skull base, an additional consideration is a chondrosarcoma, which may show similar T2-hyperintense signal but often less avid contrast enhancement and a more flocculent pattern of internal calcification.37 As with IOVMs in other locations, the MR imaging appearance may be nonspecific, and CT often provides the most diagnostic utility with a characteristic internal spiculated matrix (Table 2).
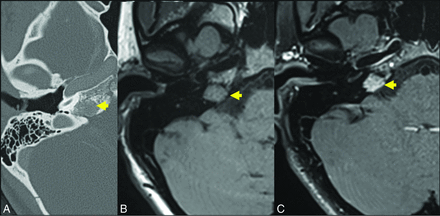
Axial T1-weighted (C) and axial T1-weighted C+ (B) demonstrate an expansile lesion centered within the left greater wing of the sphenoid, with internal T1-hypointense trabeculae and avid enhancement (yellow arrows, A and B). There is mass effect on the left orbit, with resultant proptosis. Axial T2WI (C) demonstrates that the lesion is heterogeneous but predominantly T2 hyperintense (yellow arrow, C). There is vasogenic edema in the subjacent left temporal operculum, presumably based on mass effect, a response to hypervascularity, and venous congestion. Axial CT (D) is confirmatory for the diagnosis of the IOVM, given the presence of thickened internal trabeculae (yellow arrow, D) in a characteristic pattern. The patient was treated with surgical resection. The diagnosis of IOVM was confirmed on pathology, which showed dilated thin-walled blood vessels distending and replacing medullary bone. C+ indicates with contrast. Case courtesy of Phillip Chapman, MD, Professor of Radiology, Duke University School of Medicine.
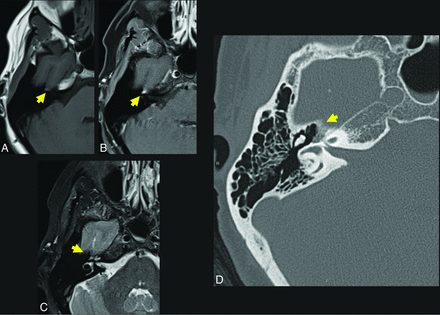
A 17-year-old girl referred for suspicion of paraganglioma identified incidentally on head CT for trauma. Axial thin-section CT (A) demonstrates a hypodense lesion centered in the right petrous apex (yellow arrow). The presence of internal trabeculae in a honeycomb pattern as well as the lesion location differentiate the correct diagnosis (IOVM of the skull base) and the suspected diagnosis of glomus jugulare paraganglioma. Axial T1 C– (B) and axial T1 C+ (C) sequences through the skull base demonstrate that the lesion is T1-intermediate (yellow arrow, B) and avidly enhancing (yellow arrow, C). A diagnosis of IOVM was made on the basis of characteristic imaging appearance. C+ indicates with contrast; C–, without contrast.
Facial Nerve
The facial nerve (FN) IOVM is the most well-recognized vascular malformation within the head and neck (Fig 7). The literature is confounded by inconsistent terminology across multiple medical disciplines and improper pathologic designation of lesions, though recent reviews have aimed to reconcile this issue.38 While most craniofacial IOVMs are incidental or cosmetically deforming, FN IOVMs are typically discovered because of related clinical symptomatology. The more common FN schwannoma may compress the nerve and cause dysfunction, whereas a venous malformation may directly infiltrate the nerve and/or cause a vascular steal phenomenon.39 Therefore, symptoms such as facial paresis or hemifacial spasm or both are much more common with FN IOVMs than the more prevalent schwannoma. When compared with facial neuritis such as Bell palsy, FN dysfunction in IOVMs is more insidious and progressive and will not spontaneously resolve.
A 57-year-old woman who presented with right facial synkinesis. Noncontrast T1WI (A) and contrast-enhanced T1WI (B) demonstrate an enhancing lesion centered at the level of the right FN hiatus (yellow arrow, A and B), with corresponding increased signal on T2WI (C, yellow arrow indicates lesion). Differential considerations included both schwannoma and IOVM of the FN. Axial (D) thin-section CT of the temporal bone demonstrates an expansile, lucent lesion at the level of the anterior genu of the FN and FN hiatus (yellow arrow, D). The internal trabeculae help to confirm the diagnosis of IOVM because schwannoma would be less likely to show internal calcification. The diagnosis of IOVM was confirmed on pathology.
The location for an IOVM is characteristically at the geniculate portion of the FN, which is not surprising given its rich venous and capillary plexus. There is a rarer occurrence elsewhere along the FN, namely at the internal auditory canal or second genu.39 FN IOVMs often infiltrate beyond the margins of the FN canal and geniculate ganglion, aiding in discrimination from facial nerve schwannoma.
CT typically reveals an expansile lesion with irregular margins centered at the geniculate fossa, demonstrating characteristic internal bony spicules or trabeculae, a honeycomb pattern, or small, needle-shaped calcifications as recently described by Yue et al.40 However, when small, an FN IOVM may fail to show typical bony densities and only widen the FN canal. Discrimination may then prove difficult between an IOVM and neoplasm, most commonly a schwannoma in this location.41 MR imaging typically reveals a mass of T1-hypointense or -isointense and T2-hyperintense signal that may be heterogeneous depending on degree of bony matrix. Typically, an FN IOVM appears as a patchy or geographic focus of enhancement, whereas a schwannoma may appear ovoid or tubular. Particularly when lesions are small, differentiation may be very difficult, and ultimately both CT and MR imaging are frequently required in this differential diagnosis.
Definitive treatment of an FN IOVM is surgical, especially if there is documented progression in FN dysfunction. Early detection and shorter duration of symptoms lead to a greater chance of FN function following surgery. The surgical approach may be subtemporal or via the middle cranial fossa, with the goal being FN identification and preservation. The lesion is most often encountered as a deep red, sinusoidal, and hemorrhagic mass with a honeycomb or spongy consistency.40 Depending on lesion location and surgical skill and resources, interposition nerve grafting can be performed to improve clinical outcomes.
CONCLUSIONS
IOVMs are rare-but-underdiagnosed entities that have been miscategorized as hemangiomas in the literature across multiple disciplines. In the head and neck, these are found in the skull, skull base, and facial skeleton, with lesions at the geniculate ganglion (incorrectly termed “facial nerve hemangiomas”) perhaps the most widely known. While a seemingly aggressive appearance may be of concern to patients, clinicians, and radiologists, a generally indolent clinical course and the presence of near-pathognomonic internal bony spicules on CT should suggest this diagnosis.
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
Indicates open access to non-subscribers at www.ajnr.org
References
- Received February 10, 2022.
- Accepted after revision May 10, 2022.
- © 2022 by American Journal of Neuroradiology