Abstract
BACKGROUND AND PURPOSE: There are 3 main types of spinal CSF leaks, and the imaging appearances are well-reported. Specific patient demographics and spinal locations of the various types of spinal leaks are less frequently described. The purpose of this article was to stratify the various types of spontaneous CSF leaks on the basis of age, body mass index, and spinal level.
MATERIALS AND METHODS: Retrospective review was performed for all patients with spontaneous spinal CSF leaks identified on CT myelography. Age, body mass index, and spinal CSF leak type and level were recorded.
RESULTS: Sixty-five patients (37 women and 28 men) had spinal CSF leaks. Type 1 CSF leaks (dural tears) were observed in 25 patients (mean age, 44.5 years; mean body mass index, 24.3) and were most common in the upper thoracic spine (72%), particularly at the T1–T2 level (36%). Type 2 CSF leaks (ruptured meningeal diverticula) were observed in 4 patients (mean age, 45.5 years; mean body mass index, 27.5) and were all seen in the lower thoracic spine. Type 3 CSF leaks (CSF-venous fistulas) were observed in 36 patients (mean age, 58.8 years; mean body mass index, 27.0) and were most common on the right side (72%) and in the lower thoracic spine (56%).
CONCLUSIONS: Type 1 CSF leaks occurred in younger patients with a normal body mass index, while patients with type 3 CSF leaks were relatively older and had an elevated body mass index. Type 1 leaks mostly occurred in the upper thoracic spine, and types 2 and 3 leaks mostly occurred in the lower thoracic spine.
ABBREVIATIONS:
- BMI
- body mass index
- CTM
- CT myelography
- CVF
- CSF-venous fistula
- SIH
- spontaneous intracranial hypotension
- W
- Shapiro-Wilk test statistic
Spontaneous intracranial hypotension (SIH) is typically secondary to a spinal CSF leak, and there are 3 main types: dural tears (type 1), ruptured meningeal diverticula (type 2), and CSF-venous fistulas (type 3).1 In the past several years, our awareness and detection of these spinal CSF leaks have increased secondary to novel techniques such as dynamic prone myelography for dural tears and decubitus myelography for ruptured meningeal diverticula and CSF-venous fistulas (CVFs).2⇓-4
Despite these advancements, there are many unanswered questions, including why spontaneous CSF leaks occur and in what demographics. It is known that spinal CSF leaks are more common in adults than in children and in females versus males.5,6 In 2016, Schievink et al1 reported the average patient age at presentation for the various types of CSF leaks; however, our detection of CVFs has grown exponentially since that date. Body mass index (BMI) is another feature that has not been well-described in patients with spontaneous CSF leaks. It has been established that obesity is a risk factor for cranial CSF leaks;7 however, there is minimal discussion about how BMI relates to spinal CSF leaks. To our knowledge, only 2 prior reports exist in a small number of patients, including a publication by Rosebrock et al,8 which reported ventral dural CSF leaks in patients with low BMI values, while Schievink et al9 reported BMI as it relates to CVF in the setting of morbid and super obesity. Last, a holistic evaluation of the spinal leak location of the various types of spinal leaks has been described in only small series.10 The purpose of this article was to stratify the various types of spontaneous CSF leaks on the basis of age, BMI, and spinal leak level and to determine if there are key differences in these characteristics; this knowledge can help guide physicians in determining which type of CSF leak and location is most likely to aid in the diagnostic work-up.
MATERIALS AND METHODS
Institutional review board (Kaiser Permanente Medical Center) approval was obtained, which waived the requirement for informed consent. The study population consisted of all patients with spontaneous spinal CSF leaks from August 2018 to March 2022 from a single institution. Inclusion criteria consisted of the following: 1) SIH diagnosis according to the International Classification of Headache Disorders, 3rd edition;11 2) pretreatment Bern SIH scores on contrast-enhanced brain MRIs;12 and 3) spinal CSF leak detection using dynamic prone CT myelography (CTM) for ventral dural tears and decubitus CTM for ruptured meningeal diverticula and CVFs. The spinal CSF leak level was obtained in each patient, and the levels were further subdivided into cervical spine, upper thoracic spine (T1–T6 levels), lower thoracic spine (T7–T12 levels), and lumbar spine. In the setting of dural tears, the presence or absence of a calcified disc was specified. In the setting of ruptured meningeal diverticula and CVFs, the laterality was documented. Independent reviews of the myelograms were performed by 2 neuroradiologists with 8 and 11 years of experience with myelograms.
The sex, age, and BMI at the time of CSF leak diagnosis were recorded. To identify any differences in age, BMI, and spinal leak level among the 3 types of CSF leaks, we performed homoscedastic t tests (Excel; Microsoft), and P values < .05 were considered statistically significant. This same statistical analysis was also performed on the Bern SIH scores, though it was not a main analysis of our study. Before t test calculations, the Shapiro-Wilk test (https://www.statskingdom.com/shapiro-wilk-test-calculator.html) was calculated for each data set to determine the normal Gaussian distribution, with a test statistic (W) between 0.938 and 1 regarded as a normal distribution for our data set.
Last, a brief discussion on the type of CSF leak treatment will be mentioned and whether there was improvement of clinical symptoms. A detailed analysis of the treatment outcomes is beyond the scope of this article.
RESULTS
There were 65 total patients (37 women, 28 men) with spontaneous CSF leaks (Table). The mean Bern SIH scores in the patient cohorts with types 1, 2, and 3 CSF leaks were 5.4, 5.8, and 5.9, respectively. Type 1 CSF leaks (dural tears) were observed in 25 patients (mean age, 44.5 years; mean BMI, 24.3). Type 2 CSF leaks (ruptured meningeal diverticula) were observed in 4 patients (mean age, 45.5 years; mean BMI, 27.5). Type 3 CSF leaks (CVFs) were observed in 36 patients (mean age, 58.8 years; mean BMI, 27.0). Given the small sample and insufficient power of the type 2 leaks, this cohort was excluded from all statistical analysis. Type 1 and 3 leaks demonstrated normal Gaussian distributions for patient age (W = 0.952 type 1 and W = 0.957 type 3) and BMI (W = 0.985 type 1 and W = 0.977 type 3). Patient age and BMI were statistically significant between types 1 and 3 (P < .00001 and P = .015, respectively).
Patient demographics of spinal CSF leaks
The BMIs were also compared with age- and sex-matched national BMI averages, and the range of type 1 CSF leak BMIs was 3.6–7 and 5.3–7.5, less than national average BMIs for males and females, respectively. The range of type 3 CSF leak BMIs was 0.6–4.1 and 0.1–5.6, less than the national average BMIs for males and females, respectively.13 The Bern SIH scores were not statistically significant (P = .44).
For type 1 CSF leaks, the leak level was most commonly in the upper thoracic spine (18/25, 72%), and 9/25 (36%) were at the T1–T2 level. Twenty of 25 patients (80%) with type 1 leaks had an associated calcified disc. Type 2 CSF leaks occurred at the left T9–T10, right T10–T11, left T11–T12, and right T12–L1 levels. Type 3 CSF leaks were more commonly on the right side (26/36, 72%) and usually lower thoracic (T7–T12) in location (20/36, 56%). The spinal leak level was statistically significant between types 1 and 3 (P < .0002). Representative images of each spinal leak type are seen in the Figures 1–3.
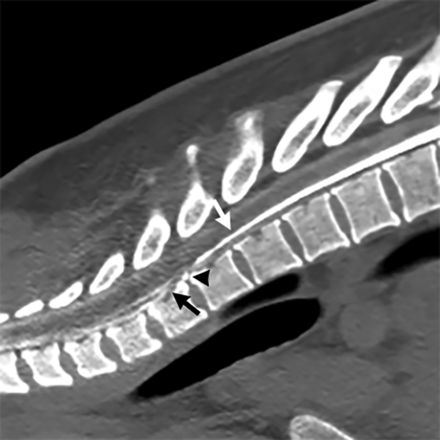
Type 1 CSF leak (dural tear): dynamic CT prone myelogram in the sagittal plane shows normal myelographic contrast in the subarachnoid space (white arrow) until there is a transition point at the T1–T2 level where there is a calcified disc (black arrowhead) that results in a split of contrast between the subarachnoid and ventral extradural spaces (black arrow).
Type 2 CSF leak (ruptured meningeal diverticulum): left decubitus CT myelogram in the axial plane shows contrast leaking from the left T9–T10 meningeal diverticulum into the neural foramen (arrow).
Type 3 CSF leak (CVF): right decubitus CT myelogram in the axial plane shows a paraspinal vein (arrows) that was contiguous with the right T10–T11 meningeal diverticulum.
Of the 25 patients with type 1 CSF leaks, all were treated with CT-guided blood and/or fibrin glue patches, and 6 patients had surgical repair of the dural leak. All 4 patients with type 2 CSF leaks were treated with CT-guided fibrin glue patches. Of the 36 patients with type 3 CSF leaks, the initial 2 were treated with surgical ligation of the CVF and the remaining 34 were treated with CT-guided fibrin glue occlusion using the technique described in another publication.14 All 65 patients had clinical improvement in symptoms.
DISCUSSION
Our study shows that age, BMI, and spinal leak level vary by spinal CSF leak type. Patients with type 1 CSF leaks (dural tears) are typically younger (mean age, 44.5 years) and have a normal BMI (mean, 24.3), while patients with type 3 CSF leaks (CVFs) are relatively older (mean age, 58.8 years) and have an elevated BMI (mean, 27.0). This easily obtained patient information could help provide guidance in the spinal CSF leak imaging pathway. Furthermore, type 1 CSF leaks were most common in the upper thoracic spine, and types 2 and 3 were most common in the lower thoracic spine.
Patients with type 1 CSF leaks had lower average BMIs than those patients with type 2 or 3 CSF leaks and also had lower BMIs than national averages. The type 1 patient characteristics match those in a smaller study that previously reported patient BMIs to be lower than the national average.8 Furthermore, a majority of type 1 CSF leaks in our study were usually secondary to calcified discs or microspurs (80%) that resulted in dural tears. The thoracic spine was the most likely location in our study (92%), and this is supported with another study.15 In our study, the upper thoracic spine at the T1–T2 level was the most common site (36%), and this high frequency of upper thoracic dural leaks was also reported in at least 1 other study.16 While thoracic disc-related leaks were observed more frequently in the upper thoracic spine, thoracic disc herniations, in general, are reported to more commonly occur in the lower thoracic spine, given that it has more mobility than the upper thoracic spine. Thoracic discs also have a high predisposition for calcification and dural tears.17,18 We speculate that 1 possible reason that CSF leaks occur is due to the fixed biomechanics of the thoracic spine and the apposition of the dura to posterior vertebral bodies, given the normal kyphotic curvature.19 In our study, patients with type 1 CSF leaks had normal BMIs, and some patients had BMIs as low as 18. We postulate that a lower BMI may be accompanied by less spinal epidural fat, thus acting as less of a protective barrier to the calcified discs, thereby resulting in a dural tear. Another publication has also proposed the same possibility,8 but further studies are needed to verify this theory.
On the other hand, type 3 spinal CSF leaks generally occurred in overweight patients. It has been suggested that elevated spinal pressure or pre-existing idiopathic intracranial hypertension may be a potential etiology in some patients with CVFs, akin to the phenomenon that occurs with skull base leaks. This elevated pressure has also been postulated in the setting of de novo CVFs that occur after successful CVF treatment, whereby the elevated pressure is driving the creation of a new fistula.20 Arachnoid granulations are closely related to paraspinal veins, and rupture of these granulations has been suggested as a possible inciting event of CVFs.21 Overweight patients have a higher susceptibility to elevated CSF pressures, possibly accounting for this arachnoid granulation rupture, and this could explain why patients with CVFs are more common in this population. While BMIs in this cohort were in the overweight category, they were slightly less compared with national averages, and we suspect that our patient population in Northern California has lower BMIs than in the rest of the country, but this information was not readily available for accurate comparison. Last, type 3 spinal leaks were most common on the right side and the lower thoracic spine. This laterality and spinal location correspond to information in another study,22 but a separate study has reported more CVFs on the left side.21
Type 2 spinal CSF leaks were the least common in our patient population. This finding contrasts with an older study that stated that type 2 leaks were the most common type of CSF leak.1 In that study, it is possible that many of these type 2 leaks may have been CVFs arising from meningeal diverticula, which may not have been well-detected because decubitus myelographic techniques for CVFs were not discovered at that time.23 In fact, CVFs were the most commonly detected CSF leak in our study, and with greater recognition of this entity, perhaps it will be the most common type of spinal leak. On the basis of the small number of patients in our study who had type 2 CSF leaks, it is difficult to make specific conclusions on age and BMI; however, the spinal levels in all 4 patients were the lower thoracic spine. This finding mirrors the most common location of CVFs, and we suspect that these 2 types of spinal leaks may have some similarities in pathogenesis.
The imaging pathway for CSF leak detection in patients with SIH varies per institution. In our spinal CSF leak program, after obtaining contrast-enhanced brain MR imaging, we perform a noncontrast total spine MR imaging with T2 fat-suppressed sequences to identify the presence or absence of an extradural collection. If there is a ventral extradural collection, we perform an ultrafast or dynamic prone CTM to identify the presumed dural tear. If the extradural collection is more eccentric on the MR imaging and there is concern for a ruptured meningeal diverticulum, we perform dynamic decubitus CTM to identify the ruptured meningeal diverticulum. If there is no extradural collection on the spine MR imaging, we perform decubitus CTM to identify the CVF.
While an imaging pathway such as this one usually identifies the specific CSF leak type and cause, determining the pretest probability of the spinal leak type based on age and BMI could potentially provide guidance in this imaging work-up. For example, type 1 spinal leaks were more common in the upper thoracic spine; therefore, an adequate Trendelenburg angle is needed when performing the dynamic myelography to ensure that the contrast flows to the upper thoracic spine. This knowledge can help minimize unnecessary scans in the lower thoracic spine. In fact, on the basis of this knowledge of type 1 leaks, we have changed our dynamic prone CTM technique. We used to inject a small volume of preservative-free iohexol contrast (Omnipaque 300; GE Healthcare) in the lumbar spine (1–2 mL) and then scan the total spine from caudal to cranial, cranial to caudal, and caudal to cranial. However, we observed that the contrast did not always traverse the upper thoracic spine, and there was often no leak in the lower spine; therefore, the patient had unnecessary radiation. Currently, we inject 3 mL of contrast with 1 acquisition of the total spine. If there is no spinal leak identified in the lower spine, we inject 2–3 mL more and then scan a more cranial part of the spine with some overlap of the normal area in 1 acquisition. We repeat this process until we identify the CSF leak, administering a maximum of 10 mL. This technique has resulted in less radiation to patients than our initial technique. Thus, understanding the pretest probability of the spinal CFS leak type and location can help with the diagnostic work-up.
The recognition of mean age and BMI in patients with spinal CSF leaks may also help when the diagnosis of SIH is equivocal. Because there is no absolute imaging test or clinical symptom to exclude the diagnosis of SIH, some patients may undergo many imaging examinations, myelograms, and treatments, even if the diagnosis of SIH and spinal CSF leak is not definitive. These demographic features of age and BMI may represent additional data points to help with decision-making in this difficult-yet-not uncommon scenario.
Our study has limitations, including its retrospective nature. Second, we did not obtain opening pressures consistently in our patients, which could help provide additional information. Nevertheless, it is known that opening pressures are normal in most patients with CSF leaks,24 and this datum point may not have added noteworthy information. Last, most of our patients were from 1 geographic state, which could result in differences in body habitus. Nonetheless, our hospital is a major tertiary referral center for our multihospital network consisting of >20 hospitals over a very large area; therefore, the population did have some geographic diversity.
CONCLUSIONS
Age and BMI may help predict the type of spinal leak in patients with SIH. In our study, we found that patients with dural tears (type 1) were typically younger and had normal BMIs, while patients with CVFs (type 3) were relatively older and had an elevated BMI. Type 1 leaks were more common in the upper thoracic spine, while type 2 and 3 leaks were more common in the lower thoracic spine. Further studies are needed to validate these results, but this information may help guide the imaging work-up in spinal CSF leak detection.
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
Indicates open access to non-subscribers at www.ajnr.org
References
- Received March 21, 2022.
- Accepted after revision April 29, 2022.
- © 2022 by American Journal of Neuroradiology