Abstract
BACKGROUND AND PURPOSE: The fornix-fimbria complex is mainly involved in emotions and memory. In brain MR imaging studies of young children, we have occasionally noted DWI hyperintensity in this region. The significance of this finding remains unclear. This study evaluated the DWI signal in the fornix-fimbria complex of children 0–2 years of age, including the frequency of signal hyperintensity and clinical context.
MATERIALS AND METHODS: Brain MR imaging of 714 children 0–2 years of age (mean, 11 months), performed between September 2018 and May 2021, was reviewed and evaluated for DWI signal changes in the fornix-fimbria. All children with available MR imaging studies including DWI were included. Children with poor image quality, poor visualization of the fornix-fimbria region, and missing medical data were excluded. Additional imaging findings were also evaluated. Demographic data were retrieved from the medical files. We compared the ADC values of the fimbria and fornix between children with and without signal changes. The unpaired 2-tailed Student t test and χ2 test were used for statistical analysis.
RESULTS: DWI signal hyperintensity of the Fornix-fimbria complex was noted in 53 (7.4%) children (mean age, 10 months). Their mean ADC values were significantly lower than those of the children with normal DWI findings (P < .05). About half of the children had otherwise normal MR imaging findings. When detected, the most common abnormality was parenchymal volume loss (15%). The most common indication for imaging was seizures (26.5%).
CONCLUSIONS: DWI hyperintensity in the fornix-fimbria complex was detected in 7.4% of children 0–2 years of age. The etiology is not entirely clear, possibly reflecting a transient phenomenon.
The fornix is a major component of the limbic system, which is involved in emotion, learning, drives, and memory.1 The fornix is a C-shaped WM tract bundle that serves as the major output of the hippocampus, connecting it to the hypothalamus and mammillary bodies as part of the Papez circuit.1,2 Most of the white fibers that constitute the fornix originate from the subicular cortex and the pyramidal cells of the hippocampus.3 These fiber tracts start at the alveus, a thin WM band located between the hippocampus and the ependymal lining of the temporal horn, and converge to form a discrete bundle called the fimbria.1⇓-3 The fimbria gradually thickens posteriorly and separates from the hippocampus beneath the splenium of the corpus callosum, forming the crus of the fornix.1⇓-3 The fimbria and fornix are in direct continuity and sometimes referred to as the fimbria-fornix complex. Indeed, the fimbria is occasionally described in anatomic texts as a component of the fornix (and designated as the fimbria of the fornix).3
Advanced MR imaging techniques including DTI have been used to delineate the structure, function, and connectivity of the limbic system, including the fimbria-fornix region. Using high-spatial DTI of the mammillothalamic region, Kamali et al4 demonstrated direct local connections of the fornix and hippocampus to the mamillary bodies and also to more distant circuits. Concha et al5 described a case series of 11 patients with temporal lobe epilepsy, 6 of whom also had mesial temporal sclerosis. Presurgical DTI of the fornix-fimbria was found to correlate with histology that showed reduced cumulative axonal membrane circumference and myelin area. Other studies have shown correlations of DTI parameters with executive function and memory.6⇓-8
Pathologies of the fornix are quite uncommon and often overlooked on MR imaging. These may include congenital abnormalities (which are extremely unusual, with only a few patients having been documented), various tumors, infections (eg, as part of herpes simplex encephalitis), multiple sclerosis, trauma, and Wernicke encephalopathy.2 In children, forniceal abnormalities are even less common; a few studies have described imaging changes in the Papez circuit in various pediatric conditions such as hypoxic-ischemic injury,9 22q11.2 deletion syndrome,10 and congenital central hypoventilation syndrome.11
While reviewing brain MR imaging studies of young children in our tertiary pediatric medical center, we have noted signal hyperintensity in DWI sequence in the fornix-fimbria complex in young children (mainly 9–12 months of age). The significance of this finding was not clear: Does it represent a normal age-related finding (possibly developmental) or perhaps an abnormality? Therefore, our aim in the present study was to assess the frequency of DWI signal changes in the fornix-fimbria complex in young children and to correlate these findings with clinical features and other imaging findings.
MATERIALS AND METHODS
This retrospective, single-center study was approved by Schneider Children's Medical Center of Israel institutional review board with a waiver of written informed consent.
Patients
Data were evaluated from 714 children, 3 days to 2 years of age (mean, 11 months), who had been referred for brain MR imaging in our tertiary pediatric medical center between September 2018 and May 2021. All children with available MR imaging studies that included DWI sequences were included in the study. Study exclusion criteria were poor image quality, including poor visualization of the fornix-fimbria region, and missing medical data, including patients’ demographics and clinical background. MR imaging studies were reviewed by 2 certified pediatric neuroradiologists (M.S.R. with 6 years’ experience and O.K. with 17 years’ experience) and assessed for DWI signal changes in the fornix-fimbria complex. The study group comprised patients in whom increased signals were noted on DWI in >1 axial section in the fimbria-fornix region (Fig 1). From the entire cohort of children who met the eligibility criteria, we arbitrarily selected children in a ratio of 2:1 who were age-matched within 1 month to the study group and who were without fimbria-fornix abnormalities (Fig 2). In addition, we evaluated fornix-fimbria DWI signal changes on MR imaging performed from January 2021 to March 2021 of 100 consecutive children 2–5 years of age (mean, 3.4 years). Again, only MR imaging studies with good visualization of the fornix-fimbria region were reviewed. Poor-quality studies were excluded.
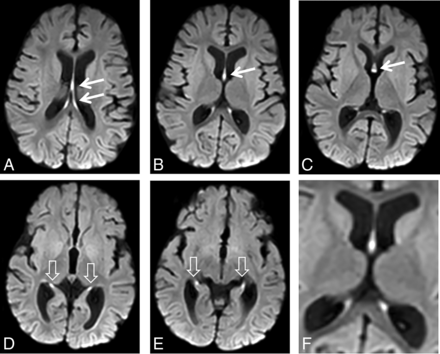
DWI signal hyperintensity in the fornix-fimbria. MR imaging study of a 7.5-month-old girl with hypsarrhythmia. Consecutive diffusion-weighted images from top (A) to bottom (E) show signal hyperintensity of the fornices (arrows) and fimbriae (arrowheads). F, A magnified view shows DWI hyperintensity involving the fornix-fimbria complex. Also notable is mild volume loss.
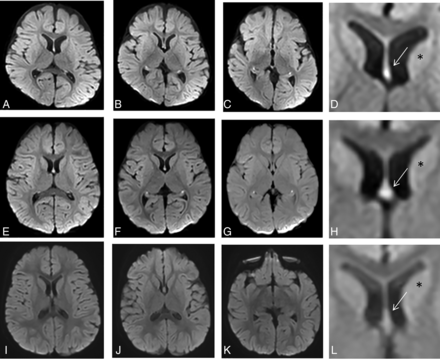
DWI signal intensity in the fornix-fimbria in a control group versus patients with high signal intensity. Two examples of children (patient 1, A–D; patient, 2 E–H) with high signal intensity detected in the fornix-fimbria on DWI, in axial slices from top to bottom. D and H, Magnified view at the level of the forniceal body (arrow) shows high signal intensity (compared with the adjacent caudate, asterisk). A third patient (I–L) with normal signal intensity of the fornix-fimbria on DWI. L, An enlarged image of the forniceal body (arrow). Usually, the DWI signal intensity of the fornix is approximately the same as that of the adjacent caudate nucleus (asterisk).
MR Imaging Technique
All children were scanned on a 1.5T Achieva scanner or a 3T Ingenia scanner (Philips Healthcare). Of the 714 MR imaging studies, 93 (13%) were performed on a 1.5T magnet.
Patients younger than 3 months of age were usually scanned without sedation (feed and wrap technique). Older children who could not remain still during the examination were sedated. The standard pediatric brain protocol included T2 FLAIR (TR/TE = 11,000/125 ms, FOV = 210 × 172 mm2, matrix = 264 × 174, section thickness = 4 mm), axial DWI using a single-shot, spin-echo-type echo-planar sequence along 2 independent axes (b-value = 0, 1000 s/mm2, section thickness = 4 mm), spin-echo T2-weighted imaging (TR/TE = 4699/98 ms, FOV = 200 × 162 mm2, matrix = 252 × 192, section thickness = 4 mm), and a sagittal 3D T1-weighted gradient-echo sequence (TR/TE = 8.1/4.7 ms, FOV = 240 × 228 mm2, matrix = 240 × 228, section thickness = 1 mm).
Image Analysis
We defined fornix-fimbria DWI signal abnormalities as hyperintensity noted on DWI in >1 axial section. We also evaluated concomitant signal changes in the fornix-fimbria on T2-weighted images in addition to other imaging findings. We placed round ROIs manually on the DWI hyperintense area seen in the body of the fornix, while trying to avoid CSF contamination. We obtained a mean ADC value for each ROI. In the fimbria, similar measurements of the ADC values were also obtained bilaterally and averaged. The ROI ranged between 0.7 and 2.8 mm2. In the control group, ROIs were positioned by anatomic guidance. We compared the mean ADC values for the abnormal fornix and fimbria between the study and control groups, measured in the same way. For the older children, 2–5 years of age, we visually evaluated the fornix-fimbria appearance on DWI.
Statistical Analysis
Descriptive statistics are reported as numbers of patients and percentages. Categoric variables were numerically coded. The unpaired 2-tailed Student t test and χ2 test were used for statistical analysis. P values < .05 were considered statistically significant.
RESULTS
In total, 714 children met study the eligibility criteria. Of them, fornix-fimbria DWI signal changes were detected in 53 (7.4%): 37 girls and 16 boys. The characteristics of these 53 patients are presented in Table 1. The patients’ ages ranged from 12 days to 22 months (mean, 10 [SD, 3] months).
Characteristics of the 53 patients with fornix-fimbria DWI signal changes
Of the 714 MR imaging studies, 93 (13%) were performed on a 1.5T magnet. Of the 53 children with DWI hyperintensity, 9 (17%) were scanned on the 1.5T magnet. A χ2 test of independence showed no statistically significant association between the MR imaging machine (1.5T or 3T magnet) and the detection of DWI hyperintensity in the fornix-fimbria, χ2 (1, n = 807) = 1.35, P = .24.
The median age in the age-matched control group was 10 (SD, 2.9) months.
Indications for performing brain MR imaging in the 53 children are shown in Table 2. The most common indication was seizures (14/53 patients, 26.5%). Among these, 6/14 (43%) patients were referred for imaging due to infantile spasms, and all of them were treated with the adrenocorticotropic hormone. There were various indications for imaging in the age-matched control group: Eleven (10%) were referred to imaging due to seizures. The proportion of patients who were referred to imaging due to seizures was significantly higher in the fornix-fimbria DWI signal-changes group, χ2 (1, n = 163) = 8.54, P < .05.
Indications for performing brain MR imaging in 53 children with DWI hyperintensity in the fornix-fimbria complexa
For about half (25/53, 47%) of the children in the study group, imaging findings other than fornix-fimbria DWI signal changes were not detected on MR imaging. Eight children (15%) had some evidence of parenchymal volume loss. Those remaining had various imaging findings such as old infarcts, hypoxic-ischemic injury, subdural collections, hydrocephalus, benign enlargement of subarachnoid spaces, and heterotopia. All the children had a normal age-appropriate sulcation and myelination pattern. None of the patients had concomitant T2-weighted signal changes in the fornix and hippocampi.
Of the 53 children with DWI hyperintensity, 4 (7.5%) had multiple studies. For 3 of them, the DWI signal changes disappeared on subsequent studies. For 1 patient who had a close follow-up 3 months later, the diffusion changes were still apparent (at 13 months of age). A second follow-up performed 3 months later did not show signal hyperintensity.
None of the 100 children 2–5 years of age had fornix-fimbria DWI signal changes.
The mean ADC value measured at the body of the fornix in the 53 children with high DWI signal intensity (684.5 [SD, 171] × 10−6 mm2/s) was significantly lower than that of the control group (832.1 [SD, 205.8] × 10−6 mm2/s), t(163) = 4.5, P < .001, unpaired t test. The mean ADC value of the bilateral fimbria in the 39 children with high DWI signal intensity (821 [SD, 103.8] × 10−6 mm2/s) was also significantly lower than that of the control group (867.2 [SD, 84.6] × 10−6 mm2/s), t(163) = 3, P < .05, unpaired t test.
DISCUSSION
Among brain MR imaging studies of our patients 0–2 years of age, DWI signal changes in the fornix-fimbria complex appeared in about 7.4%. These changes were not limited to a specific neurologic condition. However, a substantial proportion of the children (26.5%) were referred to imaging due to seizures (half with infantile spasms), substantially more than the 10% in the age-matched control group. The remaining indications for MR imaging among those with DWI signal changes in the fornix-fimbria complex were variable, ranging from sensory-neural hearing loss to torticollis and microtia. In almost half the MR imaging scans of the study group, no additional imaging findings were noted. When detected, the most common abnormality was parenchymal volume loss.
The etiology of DWI signal changes in the fornix-fimbria is not entirely clear. Furthermore, because the phenomenon appeared nonspecific (in multiple clinical scenarios), usually not in conjunction with additional imaging findings, and was confined to a specific age range (Fig 3), the basis could be physiologic/developmental rather than pathologic.
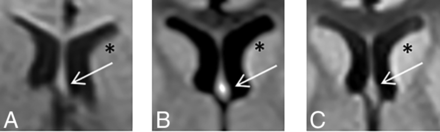
Changes in DWI signal intensity of the fornix-fimbria with time. A brain MR imaging study of a child with a low-grade cervicomedullary tumor on a DWI sequence. The body of the fornix (arrows) is shown in 3 follow-up studies performed at 3 months of age (A), 13 months of age (B), and 24 months of age (C). Fornix DWI hyperintensity is noted only at 13 months of age. Comparing the signal intensity of the head of the caudate nucleus (asterisk) shows similar signals at 3 and 24 months of age and a hypersignal at 13 months of age.
Indeed, the association between diffusivity and brain maturation has been investigated quite extensively. In a study by Sotardi et al,12 normative ADC patterns were quantified in children up to 6 years of age. ADC values decreased with time, reaching a plateau after 1.3–2.4 years. A basic pattern of the maturation process can also be elucidated from diffusion tensor imaging analyses, which show increased fractional anisotropy and a mean decrease in diffusivity in the posterior-to-anterior and central-to-peripheral directions.13⇓⇓⇓-17 These changes may correlate with a maturational process that occurs in the first 2 years of life, mainly myelination. Most interesting, other factors in the fornix appear to contribute to changes in fractional anisotropy. In addition, relatively high fractional anisotropy in neonates has been reported, though full myelination is not reached until 24 months of age, according to postmortem examination findings.18⇓-20
Whether seizures might be a contributing, perhaps modulating or potentiating, factor in DWI signal changes in the fornix-fimbria complex remains to be answered. Seizure-related changes in myelination are well-known, and accelerated myelination has been described in various conditions, including early postnatal epilepsy, hemimegalencephaly, and early Sturge-Weber syndrome.21⇓-23 Additionally, MR imaging changes in the hippocampus are occasionally seen in association with seizures and may manifest as focal swelling with increased T2-weighted and FLAIR signal intensity, with or without diffusion restriction.24 Nevertheless, distinctive bilateral features that were described in this cohort and visualized only on DWI have not been previously described.
A well-known phenomenon of symmetric signal hyperintensity in the globus pallidus, thalami, dentate nuclei, and cerebral peduncles in young children receiving vigabatrin for infantile spasms has been described quite extensively in the literature.25,26 However, in the current cohort, all the children with infantile spasms were treated with the adrenocorticotropic hormone rather than vigabatrin; overall, it seems unlikely that the signal hyperintensities described are related to antiepileptic medication.
There are several limitations to this study. A major drawback is that the described observation is limited to a single manufacturer and institution, raising concerns regarding the possibility of an artifact. Nevertheless, for a number of reasons, we believe that the observation is a true finding rather than an artifact. First, this is a unique observation limited to a very specific age group. Had it been an artifact, we would have expected it to be randomly distributed in all age groups. We also did not observe any evidence in support of an artifact, such as transgression across normal anatomic boundaries. Finally, the observation was detected on 2 magnets (1.5T, 3T), verifying that it is not limited to a single machine. Obviously, additional studies including the use of advanced techniques (such as DTI) will promote further understanding of the observed phenomenon.
In addition, we did not correlate the degree of signal changes to clinical diagnoses and were unable to correlate fornix-fimbria signal changes and long-term outcomes. For most children, we lacked follow-up MR imaging studies and were, therefore, unable to validate our assumption that the phenomenon is transient. A follow-up study that evaluates long-term prognoses can clarify this assumption.
Additionally, because the fornix passes through the ventricles, measurements of ADC values might be contaminated by adjacent CSF. Nevertheless, because we performed the measurements thoroughly in the same manner in both groups (study and control), we can assume similar contamination. We also adjusted the ROI in each MR imaging study and tried to minimize CSF contamination (Fig 4).
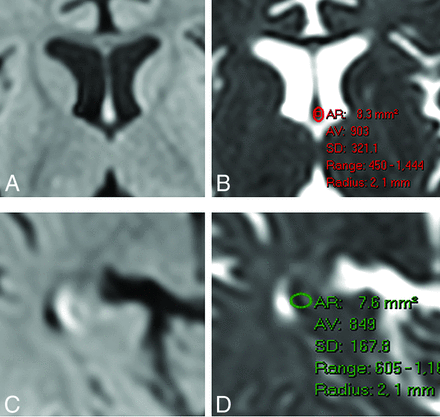
ADC measurement in the fornix (A and B) and fimbria (C and D). After detecting DWI hyperintensity on the fornix (A) or fimbria (C), we placed an ROI manually on the corresponding ADC image while trying to avoid CSF contamination and obtained the measurements. Similar measurements were performed for the control group using anatomic guidance. AR indicates area; AV, average.
CONCLUSIONS
DWI signal hyperintensity was detected in 7.4% of brain MR imaging studies of children 0–2 years of age. Seizures were the most common cause for referral to imaging, yet the observation was nonspecific and may be associated with various other conditions. The pathogenesis is not entirely clear, though it may represent a transient developmental phenomenon.
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received October 13, 2021.
- Accepted after revision January 1, 2022.
- © 2022 by American Journal of Neuroradiology