Abstract
BACKGROUND AND PURPOSE: There are limited data on the prevalence and outcome of intracranial atherosclerotic disease in patients with low-risk transient or persistent minor neurologic events. We sought to determine the prevalence and risk factors associated with intracranial atherosclerotic disease in patients with low-risk transient or persistent neurologic events.
MATERIALS AND METHODS: Participants with available intracranial vascular imaging from the Diagnosis of Uncertain-Origin Benign Transient Neurologic Symptoms (DOUBT) study, a large prospective multicenter cohort study, were included in this post hoc analysis. The prevalence of intracranial atherosclerotic disease of ≥50% was determined, and the association with baseline characteristics and DWI lesions was evaluated using logistic regression.
RESULTS: We included 661 patients with a median age of 62 years (interquartile range, 53–70 years), of whom 53% were women. Intracranial atherosclerotic disease was found in 81 (12.3%) patients; asymptomatic intracranial atherosclerotic disease alone, in 65 (9.8%); and symptomatic intracranial atherosclerotic disease, in 16 (2.4%). The most frequent location was in the posterior cerebral artery (29%). Age was the only factor associated with any intracranial atherosclerotic disease (adjusted OR, 1.9 for 10 years increase; 95% CI, 1.6–2.5). Multivariable logistic regression showed a strong association between intracranial atherosclerotic disease and the presence of acute infarct on MR imaging (adjusted OR, 3.47; 95% CI, 1.91–6.25).
CONCLUSIONS: Intracranial atherosclerotic disease is not rare in patients with transient or persistent minor neurologic events and is independently associated with the presence of MR imaging–proved ischemia in this context. Evaluation of the intracranial arteries could be valuable in establishing the etiology of such low-risk events.
ABBREVIATION:
- ICAD
- intracranial atherosclerotic disease
Intracranial atherosclerotic disease (ICAD) is judged to be causal in at least 10% of ischemic stroke cases worldwide.1,2 Prevalence is higher in non-White individuals, with ICAD as a causal mechanism in as much as 50% of ischemic strokes in people of Asian ethnicity.3 A community-based cohort estimated the prevalence of ICAD in a US population to be 9%,4 and much higher (19.0%) in people with TIA and minor stroke.5 Before the more widespread use of noninvasive neurovascular imaging, ICAD was only diagnosed with DSA following a symptomatic ischemic event and was thought to be uncommon. Today with routine noninvasive intracranial vascular imaging, we know that the prevalence of ICAD, both asymptomatic and symptomatic, is much higher.
Patients with low-risk neurologic events, defined clinically as nonmotor or nonspeech symptoms or motor or speech symptoms of a short duration of ≤5 minutes have been excluded from most epidemiologic studies of TIA and minor stroke.6,7 However, ICAD could be a marker of future vascular events in this patient population,5,8 and there is no biologic reason why ICAD should be less prevalent in this patient cohort. In the Warfarin-Aspirin Symptomatic Intracranial Disease (WASID) trial, which enrolled patients with TIA or nondisabling stroke caused by ≥50% stenosis of an intracranial artery, 18.6% of patients had an ischemic stroke during a mean follow-up of 1.8 years, with 73% of these re-occurring in the territory of the stenotic artery.9
In the present study, we evaluated the prevalence and the risk factors for ICAD and its relationship with ischemia on MR imaging in patients presenting with lower-risk transient or persistent minor neurologic events.
MATERIALS AND METHODS
Study Population
The Diagnosis of Uncertain-Origin Benign Transient Neurologic Symptoms (DOUBT) trial was an international, prospective, observational cohort study of patients with low-risk transient or persistent minor neurologic events, which enrolled 1028 patients 40 years of age or older. Detailed methods of the study have been reported previously.10 Patients were included under the following conditions: They were 40 years of age or older; were referred to a stroke neurologist for a possible minor stroke or TIA with a focal neurologic event that included either nonmotor or nonspeech symptoms of any duration, or motor or speech symptoms of ≤5 minutes duration; had an NIHSS score of ≤3 if experiencing persistent symptoms; and had undergone brain MR imaging within 8 days after symptom onset. Exclusion criteria were the following: persisting focal motor or speech symptoms for >5 minutes; symptoms of isolated monocular vision loss; history of stroke; mRS score of ≥2; serious comorbidities with an estimated life expectancy of <1 year; contraindication for MR imaging; or the examining neurologist concluding that the diagnostic criteria were definitively met for an alternative cause.
All individuals were examined by stroke neurologists before MR imaging. The final diagnosis was recorded after the completion of MR imaging and further investigations. TIA was defined clinically as a sudden loss of focal brain or ocular function of presumed vascular origin lasting <24 hours, regardless of the results of DWI.11 One-year follow-up was completed by telephone to assess recurrent stroke and death. In the case of recurrent stroke, patients were interviewed in person to confirm the event. Written informed consent was obtained for all patients. DOUBT was approved by the local institutional ethics boards at each site.
For this post hoc analysis, only patients who underwent intracranial vascular imaging via MRA and/or CTA were included.
Imaging Procedures
All patients underwent brain MR imaging per the DOUBT study protocol within 8 days of symptom onset. The CT and MR imaging manufacturers and imaging protocols varied from site to site. Acute or hyperacute infarction lesions were centrally assessed by an independent core lab using DWI, ADC, and FLAIR sequences.
For this study, intracranial and extracranial vascular images were retrospectively reviewed by 2 independent readers (a neuroradiologist and a vascular neurologist) who were blinded to the clinical symptoms and DWI results, and conflicts were resolved by a third reader.
The intracranial vasculature was assessed on CTA using reconstructed and unreconstructed images or on MRA using a high-resolution 3D TOF sequence.
We performed a dedicated analysis of the following arterial segments for evidence of ICAD: the intracranial distal ICA, MCA (M1 and M2), anterior cerebral artery (A1 and A2), posterior cerebral artery (P1 and P2), basilar artery, and intracranial segment of the vertebral arteries. Intracranial stenosis degree was evaluated using the WASID method12 by assessing the ratio between the narrowest luminal point and the normal arterial diameter before the stenosis.
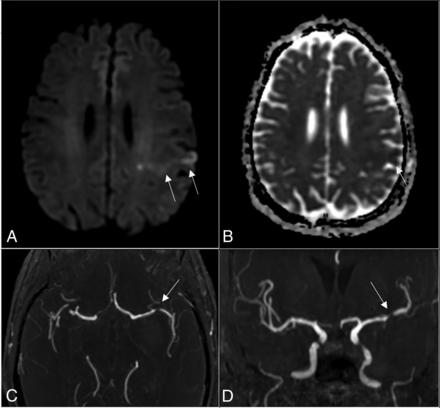
In this study, ICAD was defined as ≥50% stenosis (Figure). Asymptomatic and symptomatic ICAD were defined according to the clinical presentation and results of the MR imaging. The DWI lesion must be in the downstream territory of the affected artery to be considered secondary to ICAD, ie, symptomatic ICAD. In case of a concomitant ipsilateral extracranial arterial stenosis (only 1 case), ICAD was considered asymptomatic if the extracranial stenosis was more severe. The extracranial arterial evaluation included the common and internal carotid arteries and vertebral arteries, and stenosis was assessed using the NASCET method applied to the reformatted axial CTA images.13 Severe extracranial stenosis was defined as a stenosis of ≥50%.
A and B, DWI shows small left parietal cortical infarcts with reduced ADC (white arrows). C and D, Axial and coronal MRA views, respectively, show stenosis of >50% of the left MCA M1 segment (white arrows).
Statistical Analysis
Continuous variables were expressed as median (interquartile range), and categoric variables were expressed as frequencies. Baseline characteristics were compared between patients with and without ICAD using the Fisher exact test or Wilcoxon rank-sum test as appropriate. Interrater agreement for the presence of ICAD of ≥50% was assessed in a random sample of 50 patients who underwent MRA or CTA using the Cohen κ. We further determined the factors associated with the presence of any ICAD using multivariable logistic regression, adjusting for age, sex, and variables that were significant in the univariable analysis.
Furthermore, we compared baseline characteristics between the groups with and without DWI lesions using univariable and multivariable analyses. For the multivariable logistic regression, we adjusted for predefined variables that were either statistically significant from the univariable analysis or that have been previously shown to be associated with DWI positivity.14 These included age, sex, any motor or speech symptoms, ongoing symptoms on examination, abnormal initial neurologic examination findings, diabetes mellitus, atrial fibrillation, and ICAD. Furthermore, we repeated the regression analysis by including the variable extracranial stenosis in patients with available extracranial imaging (n = 335).
Additionally, we calculated the population-attributable risk of DWI lesions caused by any ICAD.15 To analyze the utility of intracranial arterial assessment in predicting DWI lesions, we used the Akaike information criterion and Bayesian information criterion to compare information loss between statistical models with and without the ICAD variable. Statistical analyses were performed with STATA/MP 15.1 (StataCorp), and P < .05 was considered statistically significant.
Data Availability
Data related to the current study will be available from the authors on reasonable request and approval by the DOUBT Scientific Committee.
RESULTS
Of 1028 patients included in the main DOUBT study, 661 (64.3%) had intracranial vascular imaging. Of these, 213 (20.7%) were via CTA and MRA, 329 (32.0%) via MRA alone, and 119 (11.6%) via CTA alone. Vascular imaging was performed as part of the institutional clinical routine practice in 7 of 9 participating centers, all Canadian. Patients who underwent vascular imaging (n = 661) had a lower prevalence of vascular risk factors and were more frequently women compared with patients who did not undergo vascular imaging (n = 367) (Online Supplemental Data). No differences in patient demographics were noted between patients who underwent CTA or MRA alone, and similar detection rates of ICAD were observed in both modalities, 46/329 (14.0%) versus 35/332 (10.5%) in patients who underwent MRA and CTA, respectively (Online Supplemental Data).
Among the 661 patients (median age [interquartile range], 62 [53–70] years; 352 women [53.2%]), we identified 112 arteries with ICAD in 81 (12.3%) patients (63 patients with single ICAD and 18 with multiple ICADs), of whom 14 (2.1%) had symptomatic stenosis alone, 65 (9.8%) had asymptomatic stenosis alone, and 2 (0.01%) had both symptomatic and asymptomatic stenosis. In patients with available neck vascular imaging (n = 335), extracranial stenosis was less prevalent (6.3% [21/335] with 10 carotid and 11 vertebral stenoses) compared with ICAD (10.1% [34/335], P = .001). The study flow chart is shown in the Online Supplemental Data.
Posterior cerebral artery stenosis was the most frequent stenosis, accounting for 29.5% (33/112) of ICAD, followed by the intracranial ICA (25.0% [28/112]), MCA (19.6% [22/112]), the V4 segment of the vertebral artery (14.3% [16/112]), and the anterior cerebral artery (8.0% [9/112]). Basilar artery stenosis was the least prevalent (3.6% [4/112]).
The agreement between both raters for the presence of ICAD of ≥50% was good (κ = 0.62)
ICAD versus No-ICAD Subgroups
Patients with ICAD were older and more likely to have hypertension (Online Supplemental Data.). The presence of DWI lesions was more frequent in patients with any ICAD than in patients without ICAD (25/81 [30.8%] versus 65/580 [11.2%], P < .001). A final diagnosis of stroke mimic was higher in patients without ICAD (68.1%, 395/580) versus patients with ICAD (46.9%, 38/81). Baseline characteristics of patients with symptomatic-versus-asymptomatic ICAD are provided in the Online Supplemental Data.
Multivariable logistic regression analysis adjusted for age, sex, hypertension, history of migraine, speech disturbance, any motor or speech symptoms, and abnormal initial neurologic examination findings showed that only age was independently associated with the presence of any ICAD (adjusted OR, 1.98 per decile increase in age; 95% CI, 1.65–2.52) (Online Supplemental Data).
DWI Lesions versus No Ischemic Lesion
The incidence of DWI-positivity was 90/661 (13.6%). There were more men (60% versus 44.7%, P = .009) and patients were older in the DWI-positive group (median [interquartile range], 67 years [58–73 years] versus 62 years [53–70 years], P = .004) (Online Supplemental Data). ICAD was more prevalent in patients with-versus-without DWI lesions (25/90 [27.7%] versus 56/571 [9.8%], P < .001).
In the multivariable regression analysis of DWI-positivity, only male sex (OR, 2.09; 95% CI, 1.30–3.35), any motor or speech symptoms (OR, 2.23; 95% CI, 1.29–3.85), and ICAD (OR, 3.47; 95% CI, 1.91-6.25) were associated with the presence of DWI lesions. Other covariates (age, ongoing symptoms on examination, abnormal initial neurologic examination findings, atrial fibrillation, and diabetes mellitus) were no longer associated with DWI-positivity after adjustment. These results remained unchanged when the model included extracranial arterial stenosis as a covariate.
The attributable risk of DWI lesions from ICAD was estimated to be 18.9% (95% CI, 8.3%–28.9%). When we compared regression models, the model with the ICAD variable had the least information loss (Akaike information criterion = 497.02 versus 510.88, Bayesian information criterion = 532.97 versus 542.33 in the models with and without ICAD, respectively).
Clinical Outcomes
Among the 660 participants with available 1-year follow-up data, only 1 developed a recurrent stroke (without ICAD or extracranial stenosis) and 5 had a TIA at 1 year (1 with ICAD and 4 without ICAD). ICAD was not associated with unfavorable functional outcomes at 90 days because 3.7% (3/80) of patients with ICAD had an mRS score of ≥2 versus 2.9% (16/566) in patients without ICAD. At 1 year, those with ICAD had a nonsignificant increase in the risk of worse functional outcomes because 4% (3/75) of patients with ICAD had mRS ≥ 2 compared with 1.9% (10/531) in patients without ICAD (Online Supplemental Data). Similarly, clinical outcomes were comparable between patients with and without symptomatic ICAD (Online Supplemental Data).
The presence of DWI lesions was associated with a significant increase in the risk of any recurrent ischemic event (stroke or TIA) at 1 year (4/90 [4.4%] versus 2/571 [0.4%], P = .004) in the groups with and without DWI lesions (Online Supplemental Data). Six patients (2 with ICAD) died by 1-year follow-up. The presence of ICAD was associated with a nonsignificant higher risk of mortality (risk ratio, 3.4; 95% CI, 0.6–18.3).
DISCUSSION
In this post hoc analysis of the DOUBT study, we found a prevalence of 12.3% for any ICAD and 2.4% for symptomatic ICAD in this population with low-risk transient or persistent minor neurologic events. Our study highlights the importance of intracranial vascular imaging in identifying an ischemic mechanism for low-risk neurologic events and the presence of ICAD as a risk for having MR imaging–proved ischemia in this clinical context. One in 3 participants with ICAD had DWI lesions compared with 1 in 10 without ICAD.
Th prevalence of ICAD has been evaluated in several cohorts, but little is known regarding its prevalence in subjects with lower-risk neurologic presentations. In the general population, ICAD prevalence was estimated to be 9% in the United States,4 whereas in patients with TIA or ischemic stroke, the prevalence varied between studies, ranging from 7% to 36% in European cohorts to 22%–65% in Asian cohorts.16 The DOUBT population was a mix of patients with TIA/minor stroke and stroke mimics, which may explain the high prevalence that we found compared with the general population. The posterior cerebral artery was the most common ICAD location in our study. Previous studies reported various ICAD locations.16-18 For example, in a comparative analysis of 2 cohorts of Chinese and White patients, the authors found a predominant MCA involvement in the Chinese cohort, while there was similar involvement risk across the arterial locations in the White cohort.16
Older age was the only independent factor associated with ICAD in our study, in accordance with previous studies investigating the risk factors for ICAD in patients with minor stroke and TIA.5,8 However, hypertension and diabetes were also reported to be independently associated with ICAD in different reports.16,19
We found a lower prevalence of severe (≥50%) extracranial stenosis compared with ICAD in the subset of patients who underwent neck vessel imaging (n = 335; 6.3% versus 10.1%). Of note, in the Oxford Vascular Study, which recruited patients with TIA and minor stroke, the ICAD prevalence was double that of 50% or greater extracranial carotid stenosis (14.8% versus 7.2%).5 However, in our study, the incidence of extracranial carotid stenosis was lower (6.3%), which may be explained by a mix of stroke presentations (TIA/minor stroke and stroke mimics).
The rate of DWI-positivity in this substudy was similar to that of the main study cohort (13.5%),10 and ICAD was strongly associated with the presence of an ischemic infarct, regardless of other risk factors. Previous studies have found that neurologic symptoms at presentation were the main factors associated with DWI-positivity.10,14 Our study contributes to the existing literature by showing that the combination of intracranial vascular imaging and neurologic examination predicts the likelihood of DWI positivity more accurately. Identification of ICAD and the stroke mechanism, whether related to artery-to-artery embolism, branch atheromatous disease, and/or hypoperfusion, could significantly impact treatment decisions.20,21 A recent substudy of the Acute STroke or Transient IscHaemic Attack Treated With TicAgreLor and ASA for PrEvention of Stroke and Death (THALES) trial found a larger treatment effect of ticagrelor and aspirin in patients with ICAD compared with patients without ICAD, with a number needed to treat of 34 versus 92 in the overall THALES population.22 Although previous trials showed a higher risk with intracranial stent placement compared with medical treatment, these trials were criticized for enrolling patients with off-label stent usage.23,24 On the other hand, the Post Market Surveillance Study of the Wingspan Stent System (WEAVE) trial assessed the safety of on-label stent placement of ICAD by experienced interventionists and demonstrated a low rate of periprocedural complications and a relatively low 1-year stroke and death rate.25,26
The main DOUBT study included subjects with both true ischemic diagnosis and stroke mimics. We found a higher prevalence of ICAD in individuals with a final diagnosis of ischemic events versus mimics. Thus, ICAD could serve as a marker suggesting a higher risk of an underlying ischemic etiology.
In this study, ICAD was not associated with a higher risk of recurrent ischemic events.27,28 This finding might be explained by the inclusion of patients with low-risk neurologic events and the early institution of aggressive secondary preventive therapies in these patients. Additionally, the limited sample size of this study may have underestimated any association of ICAD with poor outcome in our cohort. However, ICAD was strongly associated with DWI positivity, which were associated with a recurrent ischemic event at 1 year.
Our study has limitations. Although the main DOUBT study included 3 countries, intracranial vascular images were available from only the participating Canadian centers. We note that TOF-MRA was the only vascular imaging technique used in one-third of patients and that vessel wall imaging was not performed. TOF-MRA can result in an overestimation of the degree of intracranial stenosis. We could not determine whether the arterial stenosis was due to atherosclerosis, nonocclusive emboli, or inflammatory disease. Furthermore, we could not assess the prevalence of ICAD by racial-ethnic origin because these details were not collected. The main study did not include vascular imaging; therefore, no modification in preventive treatment was made according to the presence of ICAD. The rate of recurrent ischemic events was low overall; thus, the association of ICAD with recurrent ischemic events may be underestimated. However, this study has notable strengths. The population of patients with transient or persistent minor neurologic events has been understudied for ICAD to date, and the prospective, multicenter design with near-complete follow-up supports the high-quality nature of the data.
CONCLUSIONS
In this study of patients presenting with low-risk neurologic symptoms, ICAD was seen in 12.3% and was associated with MR imaging–proved ischemia. Vascular imaging to screen for intracranial stenosis should be considered in this patient population, given its correlation with an underlying ischemic etiology.
Footnotes
The DOUBT study was funded by a grant from the Canadian Institute of Health Research.
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received September 12, 2021.
- Accepted after revision December 25, 2021.
- © 2022 by American Journal of Neuroradiology