Abstract
BACKGROUND AND PURPOSE: Isolated striatocapsular infarction occurs commonly in patients with ischemic stroke following M1 thrombectomy. We aimed to explore the correlation between CTP-derived parameters of deep venous outflow at presentation and subsequent striatocapsular infarction in a retrospective cohort of such patients.
MATERIALS AND METHODS: TTP and peak enhancement were measured on CTP-derived time-attenuation curves of the internal cerebral and thalamostriate veins bilaterally. The difference in TTP (ΔTTP) and the relative decrease in venous enhancement between the ischemic and normal sides were calculated. NCCT performed 24 (SD, 12) hours postthrombectomy was used to determine tissue fate in the caudate head, caudate body, lentiform nucleus, and internal capsule. Striatocapsular ischemia (striatocapsular infarction–positive) was defined as infarction and striatocapsular injury as either infarction, contrast enhancement, or hemorrhagic transformation in ≥1 of these regions. A striatocapsular ischemia score was calculated (0 = no ischemic region, 1 = 1 ischemic region, 2 = ≥2 ischemic regions).
RESULTS: One hundred sixteen patients were included in the analysis. Sixty-one patients had striatocapsular infarction (striatocapsular infarction–positive). The mean thalamostriate ΔTTP was 1.95 (SD, 1.9) seconds for patients positive for striatocapsular infarction and 0.79 (SD, 2.1) for patients negative for it (P = .010). Results were similar for striatocapsular injury. The mean thalamostriate ΔTTP was 0.79 (SD, 2.1), 1.68 (SD, 1.4), and 2.05 (SD, 2) for striatocapsular infarction scores of 0, 1, and 2, respectively (P = .030).
CONCLUSIONS: CTP-derived thalamostriate ΔTTP is an excellent surrogate marker for striatocapsular infarction in patients post-M1 thrombectomy. The novel approach of extracting venous outflow parameters from CTP has numerous potential applications and should be further explored.
ABBREVIATIONS:
- DT
- distance from the carotid T to the thrombus
- ICC
- intraclass correlation coefficient
- IVT
- intravenous thrombolytic
- LSAs
- lenticulostriate arteries
- SCAs+
- ASPECTS-based striatocapsular involvement
- SCI
- striatocapsular infarction
- SCInj
- striatocapsular injury
- SCIs
- striatocapsular ischemia score
The lenticulostriate arteries (LSAs) are a collection of small, deep perforating arteries arising most commonly from the M1 and supplying the basal ganglia and superior part of the internal capsule. They are commonly considered as end arteries without reliable anastomoses or collateral supply.1⇓-3
Isolated striatocapsular infarction (SCI), resulting from simultaneous occlusion of the ostia of multiple LSAs, is a common occurrence in patients who have had successful endovascular reperfusion of the target M1 segment occlusion.4⇓-6 In these patients, a rich leptomeningeal collateral supply maintains cortical and subcortical WM viability, while the deeper striatocapsular territory has infarction. Postulated mechanisms are the lack of striatocapsular collateral supply, lack of reperfusion to occluded perforators during clot retrieval, and a low ischemic threshold of basal ganglionic gray matter. Individual vascular anatomic variants have also been shown to determine the fate of striatocapsular tissue.7 The exact site of M1 occlusion as measured by the distance from the carotid T to the thrombus (DT) independently predicts the involvement of the LSAs and subsequent SCI as recently reported.8
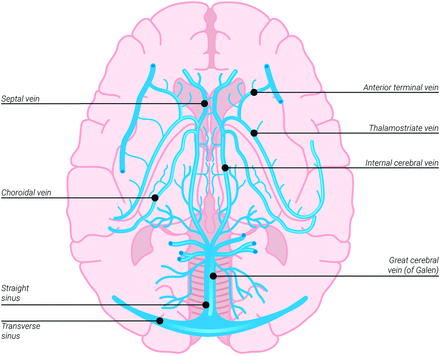
Venous outflow of the striatocapsular region relies mainly on paired thalamostriate veins, which drain into the straight sinus through paired internal cerebral veins and the great cerebral vein of Galen (Fig 1). Measuring flow parameters within these deep venous channels may be useful for tissue prognostication in patients with stroke and an M1 occlusion.
Illustration of deep cerebral venous drainage. The thalamostriate and septal veins drain into paired internal cerebral veins, which, in turn, drain into the great cerebral vein of Galen, the straight sinus, and the transverse sinuses. Also noted are the anterior terminal veins that drain into the thalamostriate veins.
CTP is used extensively to determine the eligibility of patients with anterior circulation acute ischemic stroke for mechanical thrombectomy worldwide. Currently, CTP postprocessing algorithms focus on the voxel-based analysis of cerebral parenchymal perfusion data to produce maps of the infarct core and penumbra. However, data on flow within the cerebral veins may also be extracted from the CTP images. Analysis of these widely available data enables direct, dynamic assessment of cerebral venous flow.
In this study, we aimed to explore the correlation between CTP-derived parameters of venous outflow in the deep cerebral veins and SCI in a cohort of patients with stroke following acute M1 thrombectomy.
MATERIALS AND METHODS
Study Subjects
We retrospectively reviewed all patients who had undergone mechanical thrombectomy for acute ischemic stroke with M1 occlusion and had CTP performed in the acute phase from January 2018 to December 2021 in the Rabin Medical Center. We retrieved demographics and relevant clinical data including age, sex, vascular risk factors, NIHSS at admission, intravenous thrombolytic (IVT) treatment, procedural complications, mRS score, and mortality at 90 days.
Imaging data included NCCT, CTA, and CTP from admission and NCCT that was performed 24 (SD, 12) hours postthrombectomy.
Imaging Acquisition
All study images were acquired using the same multidetector row scanner (Brilliance iCT 256 Slice CT Scanner; Philips Healthcare) including NCCT, CTA, and CTP. CTP was acquired as a 60-second cine series beginning immediately after a power injection of 40 mL of contrast at 5.2 mL/s. The scan was performed in non-Jog mode with a scan slab of 8 cm. The lower edge of the FOV was positioned at the sella turcica. The scan consisted of 30 cycles with an intercycle delay of 2 seconds. Imaging parameters included 80 kV(peak), 100 mAs, and an 0.4-second rotation time.
Imaging Analysis
Admission NCCT images were reviewed by an experienced stroke neurologist (S.P.) who determined the ASPECTS for each patient. Involvement of at least 1 striatocapsular region (caudate head, lentiform nucleus, or internal capsule) on the ASPECTS score (SCAs+) was recorded.
CTA images were reviewed by a trained neuroradiologist (M.F.) who verified M1 occlusion and assessed the cerebral collateral status using the Tan collateral score.9 He additionally reviewed postthrombectomy DSA images and recorded the modified TICI score. Successful reperfusion was defined as a modified TICI score of ≥2b. The DT on the coronal MIP (section thickness, 10 mm) was also measured for each patient.
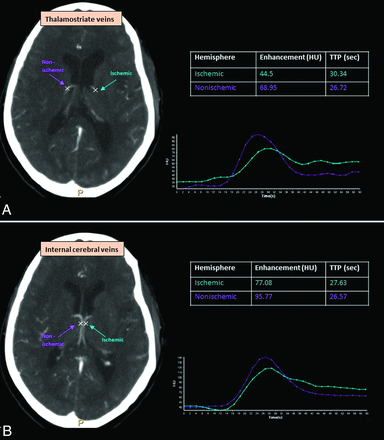
CTP data were postprocessed with the Brain Perfusion application of the IntelliSpace Portal (Philips Healthcare). All CTP scans underwent automated 3D correction for head movement during the CTP acquisition before they were analyzed. Suboptimal scans due to either excessive motion or inadequate contrast injection were excluded. Total penumbra and core volumes were automatically calculated by the application. Mean relative CBF and relative CBV of the lentiform nucleus and caudate head were measured using a manually drawn ROI. Venous outflow parameters were extracted from the CTP data by a trained neurologist (K.P.), blinded to postadmission imaging. After the bilateral internal cerebral and thalamostriate veins were visually identified on axial images of the time MIP CTP images, a circular ROI was manually placed over these veins. The software automatically detects the voxel with highest peak enhancement within this user-defined ROI and presents the time-attenuation curve and other parameters for the voxel. Measurements of TTP in seconds and peak enhancement in Hounsfield units were recorded (Fig 2). For each patient, the difference in TTP (ΔTTP = TTP ischemic hemisphere–TTP normal hemisphere) and the relative decrease in venous enhancement (enhancement ischemic hemisphere–enhancement normal hemisphere/enhancement normal hemisphere) were calculated for both internal cerebral and thalamostriate veins.
Measurement of venous flow parameters on CTP: vein selection and time-attenuation curves. A, Thalamostriate veins. B, Internal cerebral veins.
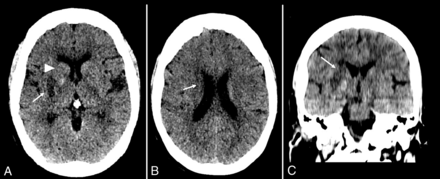
Images of NCCT performed 24 (SD, 12) hours postthrombectomy were reviewed by a second trained neurologist (J.N.) at a window width of W:40; L:40 to determine tissue fate within the 4 striatocapsular regions drained by the internal cerebral and thalamostriate veins, including the caudate head, caudate body, lentiform nucleus, and internal capsule. Caudate and lentiform nuclei infarction was evaluated on axial NCCT images. The internal capsule was evaluated shortly before the fiber tracts passed through the GM bridges between the caudate and the lentiform nucleus on coronal-reformatted NCCT images.10 Each of these regions was classified as normal, infarcted, contrast-enhanced, or with hemorrhagic transformation. A region was labeled as infarcted or contrast-enhanced when >10% of that region was involved; otherwise, it was labeled as normal (Fig 3). When available, a repeat NCCT performed from 48 hours to 7 days postthrombectomy was additionally reviewed to better define the tissue status on the 24-hour NCCT. Hemorrhagic transformation was defined as an enhanced lesion with significant mass effect, a rim of hypoattenuation, and/or when significant hyperattenuation was still evident on a repeat NCCT performed 48–72 hours postthrombectomy.11
NCCT of a patient after right. M1 thrombectomy. Infarcts involving the putamen (A, arrow), caudate body (B, arrow), and internal capsule (C, arrow) with no involvement of caudate head (A, arrowhead). SCIs of 2.
Ischemic damage was recorded separately for each of the above 4 striatocapsular regions (0 = normal, 1 = ischemic). Striatocapsular infarction–positive (SCI+) was defined as isolated infarction in at least 1 of these regions. Striatocapsular injury–positive (SCInj+) was defined more broadly as either infarction, contrast enhancement, or hemorrhagic transformation in at least 1 of these regions. A striatocapsular ischemia score (SCIs) was also calculated for each patient (0 = no ischemic region, 1 = 1 ischemic region, 2 = ≥2 ischemic regions).
Thirty CTP scans and 50 NCCT scans were independently reviewed by a third experienced stroke neurologist (S.P.) to assess interrater reliability.
Statistical Analysis
Statistical analyses were performed using SPSS Statistics for Windows, Version 25.0 (IBM). Qualitative data were represented as frequencies and percentages; the Pearson χ2 test was used for comparison of baseline characteristics, treatment (IVT), and clinical outcomes (mortality, intracerebral hemorrhage); the t test was used for comparison of treatment (arrival times) and clinical outcomes (mRS); 1-way ANOVA analysis was used for comparing intergroup differences of CTP parameters; and ORs were calculated by binary univariant logistic regression analysis to quantify the association between CTP-derived venous outflow parameters and SCI as well as between DT and SCI. Normality distribution of CTP parameters was assessed using the Kolmogorov-Smirnov test. A secondary multivariable logistic regression analysis for SCI was performed to adjust for confounders. Results were considered significant at a level of P < .05. Interrater reliability was calculated with intraclass correlation coefficients (ICCs) from 2-way ANOVA analyses that were derived to compare readers.
RESULTS
A total of 123 patients were reviewed. Two patients were excluded from the analysis due to simultaneous acute ischemia involving the contralateral hemisphere, and 1 patient was excluded due to lack of 24-hour postthrombectomy NCCT. Four additional patients were excluded due to excessive motion or inadequate contrast injection. A total of 116 patients were included in the analysis. A repeat NCCT from 48 hours to 7 days postthrombectomy was available in 43/116 (37%) patients. The internal cerebral veins were clearly demonstrated bilaterally on CTP images in all study patients. In 7/116 (6%) patients, the thalamostriate veins were not demonstrated in a sufficient quality to extract venous flow data due to variant anatomy and gross section thickness of the time MIP CTP images; for those patients, only the internal cerebral vein data were analyzed.
Demographic, clinical, and procedural data of the 116 patients included in the analysis are presented in the Online Supplemental Data. The median age was 79 years (interquartile range, 68–86 years); 49 patients were women (54.4%). There was no significant difference between patients with SCI and those without in either age, sex, vascular risk factors, NIHSS, IVT treatment, procedural complications, mRS score, or mortality at 90 days.
Baseline imaging characteristics at admission are presented in the Online Supplemental Data. The total ASPECTS was not significantly different among groups, but a higher proportion of SCAs+ was found in the SCI+ and SCInj+ groups, 24 (39.3%) and 37 (43%), respectively, compared with 4 (13.8%) in the SCI-negative (SCI–) group (P = .014). The CTA Tan collateral score was similar among the groups.
Additional CTP parameters are presented in the Online Supplement Data. The CTP-derived core volume was higher in the SCI+ group (18.8 [SD, 24] cm3) compared with the SCI– group (7.3 [SD, 8.2] cm3, P = .018). Relative CBF in the lentiform nucleus and caudate head was decreased in the SCI+ group compared with SCI– group; relative CBV was significantly decreased in the lentiform nucleus of the SCI+ group compared with SCI− group, but not in the caudate head nucleus.
Correlation between Venous Outflow Parameters and SCI
Of 116 patients included in the analysis, 61 patients were found to have isolated striatocapsular ischemia in ≥1 of the striatocapsular regions (SCI+), and 29 patients had no SCI at all (SCI–). Patients from these 2 groups were included in the main SCI analysis.
Twenty-six additional patients had striatocapsular regions with either contrast enhancement or hemorrhagic transformation. These patients together with the patients with SCI+ were defined as the SCInj+ group and analyzed separately.
The delay in venous outflow in the thalamostriate vein, ipsilateral to the occluded MCA, was significantly higher in patients with SCI+ than in patients in the SCI– group. The mean ΔTTPSCI+ was 1.95 (SD, 1.9) seconds compared with ΔTTPSCI– of 0.79 [SD, 2.1] seconds (P = .01). A trend toward higher mean ΔTTP in the internal cerebral vein in the SCI+ compared with SCI– group could also be seen, but statistical significance was not reached. No significant difference was found between the groups in relative enhancement (relative peak enhancement in the ischemic hemisphere) of either the thalamostriate or internal cerebral veins.
Analysis of patients with SCInj+ showed similar results with a mean ΔTTP in the thalamostriate vein of 1.95 (SD, 2.2) seconds compared with 0.79 (SD, 2.1) seconds in patients without any SCInj (P = .014).
A higher ΔTTP in the thalamostriate vein was significantly correlated with SCI (OR, 1.412; 95% CI, 1.072–1.86; P = .014) and SCInj (OR, 1.345; 95% CI, 1.052–1.719; P = .018). These results are presented in Tables 1 and 2. As previously reported,8 the DT was significantly correlated with SCI+ in our cohort (OR, 1.138; 95% CI, 1.058–1.223; P = .001), but the OR was lower than for ΔTTP.
CTP-derived venous outflow parameters in SCI and injurya
Correlation between delayed TTP and SCI or injury
Analysis of the SCIs as an ordinal score between 0 and 2, presented in Table 3, showed a direct correlation between ΔTTP and the SCIs, with mean ΔTTPs of 0.79 (SD, 2.1) seconds, 1.68 (SD, 1.4) seconds, and 2.05 (SD, 2) seconds for a SCIs of 0, 1, and 2, respectively (P = .030).
Thalamostriate ΔTTP and SCIs
We also analyzed the correlation between venous outflow parameters and infarction of each of the 4 striatocapsular regions separately. The correlation between higher ΔTTP and infarction was statistically significant for the caudate body and lentiform nucleus, whereas the caudate head and internal capsule showed only a trend. Results are presented in the Online Supplemental Data.
Interrater Reliability
The ICCs for the reviewed imaging parameters were 0.81 (95% CI, 0.57–0.92) for SCI on NCCT, 0.95 (95% CI, 0.91–0.98) for internal cerebral TTP, 0.95 (95% CI, 0.90–0.98) for thalamostriate TTP, 0.90 (95% CI, 0.82–0.95) for internal cerebral enhancement, and 0.93 (95% CI, 0.85–0.97) for DT on CTA. These high values represent either good (≥0.75) or excellent (≥0.9) agreement between readers for these parameters.12 The ICC was only 0.39 (95% CI, 0.05–0.65) for thalamostriate enhancement, indicating a low reliability for this parameter in our analysis.
Control of Potential Confounding Factors
Results of multivariable logistic regression analysis for SCI adjusted for age, sex, admission ASPECTS-based striatocapsular involvement, successful reperfusion, collateral score, and IVT are presented in the Online Supplementary Data. Higher thalamostriate ΔTTP remained significantly correlated with SCI (OR, 1.511; 95% CI, 1.102–2.072; P = .01). SCAs+ was a potential confounding factor and correlated with SCI (OR, 2.895; 95% CI 0.97–8.644; P = .057). However, in a secondary univariate analysis including only patients without striatocapsular involvement (SCAs–, n = 62), ΔTTP remained significantly correlated with SCI, with a mean ΔTTPSCI+ of 1.91 (SD, 2.07) seconds compared with ΔTTPSCI– of 0.89 (SD, 1.86) seconds (P = .048).
DISCUSSION
Our study establishes a significant association between delay in deep venous outflow in the acute phase of MCA ischemic stroke and subsequent SCI after M1 thrombectomy. Accumulating evidence suggests that venous outflow may be an independent modulator of stroke evolution and clinical outcomes. Formation of microthrombi in venules distal to a cerebral arterial occlusion and a “venous steal” phenomenon possibly modulate ischemic cerebral tissue fate and may explain the failure of reperfusion despite successful recanalization.13⇓⇓-16 Incorporating venous outflow into collateral status assessment in patients having undergone thrombectomy has recently been shown to improve the prediction of clinical and radiologic outcomes.17
The evaluation of cortical venous outflow patterns in acute ischemic stroke through neuroimaging surrogate markers such as opacification of cortical veins on monophasic or multiphase CTA18,19 or delayed cortical vein filling on dynamic CTA20 has been recently reported. Favorable cortical venous outflow patterns have been associated with distal vessel occlusion, good baseline collaterals, successful reperfusion, decreased infarct edema, and good clinical outcome.18⇓⇓⇓⇓-23 Administration of IVT was strongly associated with the presence of favorable venous outflow profiles in patients before endovascular thrombectomy.24 However, these neuroimaging markers have focused on the superficial cerebral venous system, which drains only cortical and juxtacortical structures. The impact of deep venous outflow on striatocapsular tissue survival has not yet been explored.
Here we present a novel approach that uses widely available CTP data to quantitate deep venous outflow as a surrogate marker for SCI. In our cohort of patients having undergone M1-thrombectomy, a higher delay in the TTP of the time-attenuation curve in the thalamostriate vein ipsilateral to an M1 occlusion (thalamostriate ΔTTP) was significantly correlated with infarction of the caudate body and lentiform nucleus separately and the striatocapsular region as a whole. Moreover, a higher thalamostriate ΔTTP was directly correlated with a larger extent of striatocapsular ischemia represented by the striatocapsular ischemia score. These results indicate that delayed thalamostriate venous flow is an excellent surrogate marker for striatocapsular ischemia. The ORs for the correlation of thalamostriate ΔTTP with SCI was higher than that of the recently reported DT8 in our cohort of patients, indicating that thalamostriate ΔTTP may be more accurate than DT as a surrogate marker for SCI.
Sparing of the lateral LSAs according to preprocedural digital DSA and asymmetric dilation of LSAs following successful thrombectomy on MRA have also been shown to predict favorable outcome following M1 thrombectomy.25,26 However, thalamostriate ΔTTP derived from CTP may be a superior surrogate marker for SCI because it provides equivalent information in a noninvasive manner and at an earlier preprocedural stage.
In our study, 2 independent raters visually identified and manually marked the bilateral internal cerebral and thalamostriate veins on axial images of the CTP scan to produce measurements of TTP and peak enhancement. These veins were easily detectable on CTP images of most of our patients. Interrater agreement was excellent for both thalamostriate and internal cerebral TTP (ICC = 0.95) and for internal cerebral enhancement (ICC = 0.899). This finding supports the strength and reliability of our novel approach to extract venous outflow parameters from CTP raw data. Indeed, interrater agreement for thalamostriate enhancement was poor, making this parameter unreliable for the analysis of deep venous outflow. This issue is probably due to the smaller diameter and high tortuosity of this vein combined with measurement of absolute peak enhancement on CTP images being highly angle-dependent. Subsequently, patient positioning may also affect this parameter.
Our results indicate that internal cerebral venous outflow is less accurate than thalamostriate outflow as a surrogate marker for SCI. While the thalamostriate veins drain the striatocapsular region exclusively, the internal cerebral veins receive several other major tributaries including the anterior septal veins, lateral direct veins, medial atrial veins,27 and choroidal veins, which drain extra-striatocapsular cerebral tissue. This process probably dampens the effect of striatocapsular ischemia on the time-attenuation curve of the internal cerebral veins. Most interesting, thalamostriate ΔTTP was highly associated with infarction of the caudate body and lentiform nucleus but less associated with caudate head infarction. This association is probably because the caudate head is drained separately by the anterior caudate vein, which enters the thalamostriate vein beyond the point where the time-attenuation curve was measured (Figs 1 and 2). Association of internal capsule infarction with thalamostriate ΔTTP also did not reach statistical significance. We hypothesize that this finding may be due to the challenge in assessing internal capsule infarction using NCCT, resulting in limited reliability. Of note, despite high anatomic variance of the thalamostriate vein tributaries, the thalamostriate vein itself is reportedly present bilaterally in >92% of patients and consistently drains most of the striatocapsular territory.28 This finding further supports the role of the thalamostriate vein as a robust imaging marker of striatocapsular drainage.
These intriguing interactions point to the enormous potential of this novel approach to extract dynamic cerebral venous outflow data with high temporal resolution from CTP images. Using the same technique, one may explore venous outflow parameters not only in acute ischemic stroke but also in healthy subjects and in patients with other acute or chronic cerebrovascular disease states. Further research may allow semi- or even fully-automatic analysis of deep venous outflow patterns in these situations.
Currently used multiphase CTA provides dynamic, high-spatial-resolution images of the cerebral vasculature. However, imaging acquisition is performed at only 3 different time points after contrast injection, and time-resolved assessment of cerebral blood flow is limited. In contrast, CTP raw data provide low spatial resolution but contain multiple time points (usually >15) and allow thorough analysis of flow parameters from the time-enhancement curve. Because our study was primarily a proof-of-concept study, we measured only TTP and peak enhancement. These parameters were the most intuitive, reproducible, and simple to extract. Additional parameters such as arrival time, wash-in time, or ascending slope may add more information and should be the subject of future analyses.
The clinical impact of SCI in the setting of successful M1 thrombectomy is uncertain. While previous studies reported pretreatment SCI to be associated with higher rates of hemorrhagic transformation, worse dysfunction and disability at discharge, and longer hospitalization,29 more recent studies have reported that it does not have a significant impact on clinical outcome.30,31 In our cohort, functional status indices at 90 days including mean mRS and the proportion of patients with good functional outcome (mRS ≤ 2) were not significantly different between patients with SCI+ and SCI– (Online Supplemental Data). This result possibly indicates that isolated SCI is not an important determinant of clinical long-term prognosis and should not directly affect the decision on thrombectomy in such patients. Nevertheless, our results are highly relevant as a proof-of-concept for the use of CTP-derived venous outflow parameters for tissue prognostication. Further studies using the same concept to explore both superficial and deep venous drainage in larger-territory MCA infarctions would most likely lead to a more significant clinical correlation.
Our study has several limitations. The patient cohort was relatively small, including only 116 patients. Data were collected retrospectively, which may introduce bias. The use of a single CT scanner type and postprocessing software may limit the generalizability of our findings. Deep cerebral veins were visually identified and manually rather than automatically marked on CTP images. Tissue fate in the striatocapsular region was determined by NCCT rather than MR imaging, due to low availability of this technique in our institution. SCAs+ on admission NCCT was common in our study and correlated with eventual SCI, raising the possibility that delayed venous outflow on CTP represents the result of early infarction rather than the cause of it. However, in a multivariable analysis that controlled for admission SCAs+ and in a secondary analysis that excluded patients with SCAs+ altogether, the correlation between thalamostriate ΔTTP and SCI+ remained significant, excluding this possibility. Finally, the use of ΔTTP as a single tissue-prognostication tool is limited by a significant overlap in ΔTTP distributions between the SCI– and SCI+ groups. However, we present a simple technique with excellent interrater reliability, and the statistical power of our findings is high. These support the high reproducibility and generalizability of our results.
CONCLUSIONS
CTP-derived thalamostriate ΔTTP is an excellent surrogate marker for SCI in patients post-M1 thrombectomy. The novel approach of extracting venous outflow parameters from CTP has numerous potential applications and should be further explored.
Footnotes
S. Peretz and K. Pardo contributed equally to this work.
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received July 12, 2022.
- Accepted after revision September 8, 2022.
- © 2022 by American Journal of Neuroradiology