Abstract
BACKGROUND AND PURPOSE: Duchenne muscular dystrophy is an X-linked disorder characterized by progressive muscle weakness and prominent nonmotor manifestations, such as a low intelligence quotient and neuropsychiatric disturbance. We investigated WM integrity in patients with Duchenne muscular dystrophy using DTI.
MATERIALS AND METHODS: Fractional anisotropy and mean, axial, and radial diffusivity (DTI measures) were used to assess WM microstructural integrity along with neuropsychological evaluation in patients with Duchenne muscular dystrophy (n = 60) and controls (n = 40). Exon deletions in the DMD gene were confirmed using multiplex ligation-dependent probe amplification. Patients were classified into proximal (DMD Dp140+) and distal (DMD Dp140–) subgroups based on the location of the exon deletion and expression of short dystrophin Dp140 isoform. WM integrity was examined using whole-brain Tract-Based Spatial Statistics and atlas-based analysis of DTI data. The Pearson correlation was performed to investigate the possible relationship between neuropsychological scores and DTI metrics.
RESULTS: The mean ages of Duchenne muscular dystrophy and control participants were 8.0 ± 1.2 years and 8.2 ± 1.4 years, respectively. The mean age at disease onset was 4.1 ± 1.8 years, and mean illness duration was 40.8 ± 25.2 months. Significant differences in neuropsychological scores were observed between the proximal and distal gene-deletion subgroups, with more severe impairment in the distal-deletion subgroup (P < .05). Localized fractional anisotropy changes were seen in the corpus callosum, parietal WM, and fornices in the patient subgroup with Dp140+, while widespread changes were noted in the Dp140– subgroup. The Dp140+ subgroup showed increased axial diffusivity in multiple WM regions relative to the Dp140– subgroup. No significant correlation was observed between clinical and neuropsychological scores and diffusion metrics.
CONCLUSIONS: Widespread WM differences are evident in patients with Duchenne muscular dystrophy relative to healthy controls. Distal mutations in particular are associated with extensive WM abnormalities and poor neuropsychological profiles.
ABBREVIATIONS:
- AD
- axial diffusivity
- FA
- fractional anisotropy
- DMD
- Duchenne muscular dystrophy
- IQ
- intelligence quotient
- MD
- mean diffusivity
- MDFRS
- Muscular Dystrophy Functional Rating Scale
- MLPA
- multiplex ligation-dependent probe amplification
- RD
- radial diffusivity
- TBSS
- Tract-Based Spatial Statistics
Duchenne muscular dystrophy (DMD), characterized by mutations in the dystrophin (DMD) gene, results in absent/nonfunctional muscle dystrophin, leading to progressive muscle weakness.1 Children with DMD also have nonmotor difficulties such as a lower intelligence quotient (IQ), reading difficulties, and increased prevalence of neurobehavioral disturbances such as anxiety, autism spectrum disorder, and obsessive compulsive disorder.2⇓-4 Very few studies have attempted to study the relationship between neurobehavioral abnormalities and neuroanatomic changes.5⇓-7
The DMD gene contains multiple independent tissue-specific promoters, producing several isoforms named according to their length and splicing patterns. The isoform Dp427m is predominantly expressed in the muscles and plays a pivotal role in structural integrity of muscle fibers, the isoform Dp427c is expressed in the cerebral cortex, hippocampus and Dp427p is mainly expressed in the Purkinje cells. The Dp140 isoform is believed to be expressed in the CNS during development, while Dp71 is expressed in both the CNS and other body tissues.8,9
Neuropsychological impairment in DMD is characterized by verbal deficits with relative sparing of nonverbal domains.10 Patients with DMD with an absence of all isoforms due to distal mutations have the lowest IQ scores, while those with the absence of only the full-length isoform achieve relatively higher scores. Moreover, children with DMD lacking Dp140 isoforms demonstrate impaired verbal memory, attention, and executive function and may develop various neurodevelopmental abnormalities.3,6,11
Neuroimaging studies have revealed structural and functional brain abnormalities,6 with MR spectroscopy12 and PET13 showing metabolic derangement. Evidence is also accruing for brain regional volume differences,14 blood oxygen level–dependent signal abnormalities,14 and altered WM integrity as measured using DTI.6 Furthermore, recent imaging studies have highlighted less severe structural abnormalities in patients with retained Dp140 expression compared with those lacking it.6,7
This study aimed to comprehensively evaluate brain changes in a larger cohort of children with DMD and further it by probing group-level differences in WM abnormalities in 2 major subtypes based on Dp140 expression, using DTI as the tool. We hypothesize that patients with retained Dp140 (Dp140+) expression will have relatively preserved WM compared with that in patients with loss of the Dp140 (Dp140–) isoform.
MATERIALS AND METHODS
This prospective study included subjects (total =100, healthy controls [n = 40], and patients with DMD [n = 60]) identified at the Neuromuscular Disorders Clinic at National Institute of Mental Health and Neurosciences. The institutional ethics committee of National Institute of Mental Health and Neurosciences (NIMHANS) approved this study. Written informed consent was obtained from the parents or guardian and child.
The diagnosis was based on clinical presentation, including delayed motor milestones, proximal weakness, hypertrophied calves, markedly elevated creatine kinase levels, and the presence of deletions detected using the multiplex ligation-dependent probe amplification (MLPA) test. Right-handed boys 6–10 years of age without any coexistent medical illness, who were ambulant, attending school, and cooperative for MR imaging and neuropsychological assessment were recruited.
Muscle power was assessed using manual muscle testing according to the modified Medical Research Council Scale for Muscle Strength, and disease severity was estimated using the Muscular Dystrophy Functional Rating Scale (MDFRS).
Exclusion criteria were severe mental retardation or suspected dystrophinopathy without obvious deletions. The healthy control children matched for age/sex, education, ethnicity, and social status were recruited from the nearby schools after obtaining approval from the school education officer and necessary consent from the parents. Subjects recruited to the control group underwent a brief neurologic examination by an expert neurologist with 20 years of experience (A.N.), and children with any psychiatric or neurologic comorbidity were excluded.
MLPA
Blood samples were collected in ethylenediaminetetraacetic acid–coated vacutainers; genomic DNA was extracted using the salting out method and stored at −20°C until tested.15 The MLPA reaction was performed to screen all exons of the DMD gene using SALSA MLPA, P034, and P035 probe sets (MRC Holland). The procedure was performed according to the manufacturer’s instructions. Amplified products were separated using a 3500XL Genetic analyzer (Applied Biosystems), and data were analyzed using the Coffalyser software (https://coffalyser.updatestar.com/) with a control sample included in every run.
Genetic results pertaining to the type and location of exon deletion were analyzed, and children were classified into 3 groups: DMD Dp140+, DMD Dp140–, and controls. The expression of the Dp140 isoform was based on the universal mutation DMD data base, a French knowledge base derived from functional studies that predicted the effect of several mutations (http://www.umd.be/DMD/4DACTION/W_ISO/L).
Neuropsychological Assessment
The battery of neuropsychological tests included the Edinburgh Handedness Inventory,16 Functional Disability Inventory, Parent Form,17 Wechsler-Intelligence Scale for Children (3rd ed, WISC-III, 1991), Rey Auditory Verbal Learning Test (WHO/UCLA Version), and Memory for Designs Test.18 The WISC-III was used to measure verbal IQ, performance IQ, full-scale IQ, verbal comprehension, the Perceptual Organization Index, and the Freedom from Distractibility Index in all children.
MR Imaging
MR imaging was performed using a 3T clinical scanner (Achieva; Philips Healthcare) and a 32-channel head coil. High-resolution 3D turbo field echo T1-weighted images were acquired (TR/TE = 9.8/4.6 ms, and spatial-resolution = 1 × 1 × 1 mm). The single-shot spin-echo echo-planar DTI sequence was performed with the following parameters: TR/TE = 5000/65 ms; resolution = 2.0 × 2.0 × 2.0 mm; noncoplanar diffusion directions = 15; b-values = 0 and 1000 s/mm2; and 2 repetitions, with a total scanning time of 4 minutes 36 seconds.
DTI Data Processing and Analysis
Diffusion data analysis was performed using FMRIB Software Library tools (www.fmrib.ox.ac.uk/fsl), Version 5.0.11. Raw diffusion tensor images were preprocessed using eddy current correction for distortions. Group comparisons of DTI data were performed using Tract-Based Spatial Statistics (TBSS; http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/TBSS). DTI-derived maps (fractional anisotropy [FA], mean diffusivity [MD], axial diffusivity [AD], and radial diffusivity [RD]) were generated using the FMRIB Diffusion Toolbox (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FDT). Individual skull-stripped FA images were aligned with Montreal Neurological Institute 152 standard space using a nonlinear registration method, followed by the creation of a group mean FA skeleton by thinning mean FA volumes (FA > 0.2 overlaid with the mean FA image). The mean FA skeleton represents the centers of all tracts common to the entire group of subjects. Each subject’s aligned FA data were then projected onto the mean FA skeleton, and the resulting data were fed into voxelwise paired-sample testing. A voxel-by-voxel permutation nonparametric test (5000 permutations) was used to assess group-related differences using threshold-free cluster enhancement, which avoids using an arbitrary threshold for the initial cluster formation. In addition to FA, a similar process of nonlinear registration and voxelwise comparison was performed to determine the differences in MD, AD, and RD maps. For all tests, a null distribution was built up over 5000 permutations, and significance was tested at a P value corrected for multiple comparisons. To assess the relationship between neuropsychological test scores and each of the DTI measures, we used an FSL General Linear Model (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/GLM). Statistical analysis was performed using the FSL Randomise tool (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/Randomise/UserGuide), with age as a nuisance variable and 5000 permutations; results were considered significant at P < .01 (family-wise error–corrected).19,20
Atlas-Based Analysis of Major WM Tracts
Multiple WM ROIs were defined using the JHU-White Matter Atlas (http://neuro.debian.net/pkgs/fsl-jhu-dti-whitematter-atlas.html), which is a probabilistic atlas generated by mapping DTI data from healthy subjects to a template image. The mean diffusion metric values of each ROI for individual subjects were extracted.
Statistics
Continuous variables are expressed as mean ± SD, and categoric variables, as frequencies and percentages. Demographic and neuropsychological data were tested for normality using the Kolmogorov-Smirnov test. Comparisons were performed using an independent-samples t test or the Wilcoxon signed rank test based on the normality of the distribution of the data. SPSS, Version 21.0 (IBM) was used for statistical computation. One-way analysis of variance with multiple comparisons using a Bonferroni post hoc test was performed to evaluate the differences in neuropsychological data and WM tract diffusion metrics between age- and-sex-matched controls and subjects with DMD (proximal and distal mutations). All mean diffusion metric values of various tracts were tested for potential associations with disease duration, IQ, and Auditory Verbal Learning Test and Memory for Designs Test scores. Scores were adjusted for age using linear regression, and the resulting standard residuals were used for correlation. The Pearson correlation coefficient was computed, and the significance threshold was P < .01. The 95% confidence intervals of the estimated parameters were also computed wherever applicable.
RESULTS
The mean age of patients with DMD and controls was 8.0 ± 1.2 and 8.2 ± 1.4 years, respectively. The mean age at disease onset was 4.1 ± 1.8 years, and the mean illness duration was 40.8 ± 25.2 months. The mean MDFRS scores are shown in Table 1. Children with upstream exon 45 (1–44) and downstream exon 45 (45–79) were subgrouped as proximal DP140+ (n = 21) and distal deletions DP140– (n = 39), respectively. Fifty-eight patients were on steroid treatment with a mean treatment duration of 9.5 ± 8.6 months (range , 1–37 months). Clinical information is summarized in Table 1. Mean MDFRS scores and individual domain scores were compared between the proximal- and distal-deletion subgroups; the distal subgroup had lower MDFRS scores. A statistically significant difference in mobility and impairment domains and overall mean MDFRS score was observed between subgroups of children with DMD (proximal = 104.13 ± 7.635 and distal = 96.41 ± 11.112, P < .05). No significant differences were noted in the dose and duration of steroid treatment between the 2 subgroups. The neuropsychological examination scores and values of the WISC-III scale are summarized in On-line Tables 1 and Table 2, respectively.
Clinical characteristics and MDFRS scores
Comparison of the intelligent quotient (IQ) values of WISC-III scale between the two patient subgroups (DMD proximal and distal), and healthy controls
TBSS Results
Comparison of DTI Metrics between Children with DMD (Proximal and Distal Mutations) and Healthy Controls.
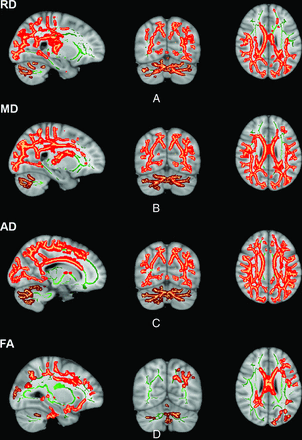
TBSS analysis comparing all patients with DMD and controls demonstrated widespread WM alterations involving both the supra- and infratentorial WM (Fig 1). There was a significant increase in MD and RD values in the WM of patients compared with controls. Focal areas of significant reduction in FA included the corpus callosum, superior longitudinal fasciculus, superior and inferior fronto-occipital fasciculus, corticospinal tract, and uncinate fasciculus in patients compared with controls.
DTI TBSS analysis comparing healthy controls with patients with DMD shows areas with significantly (P < .01, family-wise error corrected) decreased FA and increased MD and RD in patients with DMD. Green represents the WM skeleton, while red and maroon are clusters of significance.
Comparison of DTI Metrics between DMD Proximal (Dp140+) and Healthy Controls.
No significant differences were observed in any of the diffusivity parameters. FA was significantly higher in healthy controls than in patients in the bilateral fornices, the body of the corpus callosum, and parietal WM (Fig 2). Because only FA changes were seen, we used a less stringent threshold of P < .05 to evaluate trends in diffusivity metrics. We noted trends in RD maps using a less stringent threshold of P < .05 family-wise error–corrected, while other diffusivity metrics showed no changes.
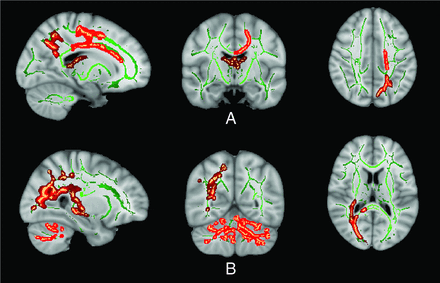
Healthy control versus DMD (distal) Dp140– TBSS results. RD (A), MD (B), and AD (C) are significantly increased in patients compared with healthy controls in both the cerebellar WM and cerebral WM with relative sparing of the frontal WM (P < .01 family-wise error–corrected). D, FA did not show any significant differences; however, the trend was noted because many areas showed FA differences at the less conservative statistical threshold of P < .05 (family-wise error–corrected). Green represents the WM skeleton, while red and maroon are clusters of significance.
Comparison of DTI Metrics between DMD Distal (Dp140–) and Healthy Controls.
A widespread increase in diffusivity indices was noted in the form of increased MD, AD, and RD involving predominantly cerebellar, occipital, and parietotemporal WM regions with relative sparing of the frontal WM (Fig 3). No significant difference in FA was noted; however, at a reduced threshold (0.01 < P <.05), lower FA was seen in the corpus callosum, corticospinal tract, left superior longitudinal fasciculus, inferior longitudinal fasciculus, and fornices.
(A) Healthy controls versus DMD (proximal) Dp140+: TBSS for FA shows significantly lower FA in the patient group in the areas highlighted in red (P < .01). No significant differences in any of the diffusivity metrics are noted. (B) DMD distal (Dp140–) versus DMD proximal (Dp140+): TBSS shows higher AD in patients with a distal mutation involving the cerebellar WM and right occipitoparietal-temporal WM. The rest of the diffusion metrics did not show any significant difference.
Comparison of DTI Metrics between DMD Distal (Dp140–) and Proximal (Dp140+) Subgroups.
Significantly higher AD values were noted in the right occipital-temporal WM, corpus callosum, and cerebellar WM in the DMD distal (Dp140–) than in the DMD proximal (Dp140–) subgroup. However, no significant difference was seen in MD, RD, and FA between these 2 subgroups (P < .01).
No significant correlations were identified between WM abnormalities and clinical severity, disease duration, or IQ.
Atlas-Based WM Analysis
Controls versus Patients with DMD (Dp140+).
Significantly reduced FA was observed in multiple tracts in patients with DMD with a proximal mutation, while MD was not found to be significantly different in this group (P < .01) (On-line Table 2).
Controls versus Patients with DMD (Dp140–).
No significant change in FA was noted in the patients with DMD with distal mutation, while higher MD values were seen in multiple tracts, listed in On-line Table 2 (P < .01).
Comparison between DMD (Dp140+) and DMD (Dp140–) Patient Sub-groups.
No significant difference in FA or MD was observed between the distal and proximal mutation subgroups (P < .01).
DISCUSSION
This study revealed lower IQ and neuropsychological abnormalities in patients with DMD, which were more severe in the distal mutation Dp140– subgroup. DTI analysis revealed widespread WM alterations in both supratentorial and infratentorial regions. Comparison of proximal and distal mutation subgroups with healthy controls using TBSS revealed significantly higher MD, RD, and AD values in the Dp140– subgroup, whereas patients in the Dp140+ subgroup demonstrated spatially localized altered FA values with no significant changes in MD. Similar findings were also detected in an atlas-based analysis of tract diffusion metrics.
DMD is a debilitating disorder characterized by progressive muscle weakness and skeletal deformities, followed by an inexorable course to severe respiratory difficulties and overall reduced life span.1 Although the clinical phenotype exemplified by muscular manifestations is the dominant phenomenon, earlier studies have identified significant nonmotor features. Neuropsychological evaluation in these patients has demonstrated impaired language and memory and executive dysfunction, which may possibly be dependent on the site of mutation.3,11,21⇓-23 Specifically, the absence of the Dp140 isoform may lead to severe neuropsychological abnormalities.3,11
Kim et al24 have reported that the brains of patients with DMD are devoid of the 427 kDa cortical dystrophin protein. While the functions of dystrophin in the brain have not been categorically described, studies have indicated that the isoform Dp427 is involved in the organization of gamma-aminobutyric acid A receptors and possibly plays a role in signaling. The functions of the shorter isoforms, Dp140 and Dp71, expressed in glial cells adjacent to the microvasculature,25 have not been elucidated. Dp140, given its expression during the early developmental stages of the brain, is purported to modulate axon guidance, transcription factor activity, and neuron differentiation.9 Postmortem studies in patients with DMD have illustrated pathologic changes such as astrogliosis, neuronal loss, heterotopia, and cortical abnormalities in patients with DMD.26,27 Functional and quantitative neuroimaging studies have revealed multiple abnormalities in DMD brains. Hippocampal and cerebellar hypometabolism has been documented on PET in DMD.13 Resting-state fMRI has elucidated reduced local synchronization of spontaneous activity in the neural networks of the motor cortex.14 Brain MR spectroscopy observations have been varied, with altered choline and phosphorus metabolite concentrations in DMD having been described in a few studies,12,28⇓-30 while no significant changes were demonstrable in another study.31 Recently, Doorenweerd et al6 reported significant brain morphometric changes along with altered WM integrity in patients with DMD. Another study from this group also showed reduced cortical perfusion in DMD, independent of cortical atrophy.5 However, visual inspection of neuroimaging is usually unremarkable.
DTI measures are surrogate markers of WM integrity, ie, directional WM integrity (FA), tissue breakdown and increased water content (AD), and axonal integrity and myelin sheaths (RD and AD). In our study, we found increased diffusivity along with reduced WM FA in patients with DMD. The Dp140– subgroup showed extensive WM diffusivity alterations with relatively localized FA changes, suggesting that diffusion changes along the direction of the major axis (AD) are commensurate with those of the minor axes (RD).32 In contrast, the Dp140+ subgroup illustrated a relatively preserved WM structure. Doorenweerd et al6 reported extensive WM changes in the diffusivity metrics and less extensive changes in FA, and alterations were more widespread and severe in the distal mutation group. Contrary to these results, another study using ROI-based analysis found only focal abnormality in the splenium of the corpus callosum.7
Our study is similar to the study of Doorenweerd et al;6 however, they had a very small sample size, studied older children with a wide age range, and performed both voxel-based morphometry and DTI analysis. Our study participants were much younger, and the age range was narrower. Another study by Fu et al7 used only 12 subjects with DMD and performed ROI-based analysis. No subgroup analysis was performed on the basis of the underlying genotype.
Multiple pathophysiologic phenomena like demyelination, WM re-organization, increased membrane permeability with excess free water, intracellular compartment changes, and glial alterations modulate diffusivity.33 Myelination abnormalities may be a candidate mechanism altering DTI metrics in patients with DMD as shown by Aranmolate et al34 in the mdx mouse model of DMD. Oligodendrocytes require dystrophin for normal maturation, which, in the case of the mdx murine model, is lacking, potentially explaining the observation of impaired myelination. Another study of the mdx mouse model showed that increased extracellular free water led to increased blood-brain barrier permeability, resulting in increased MD and decreased FA.35 This mechanism is also supported by studies that have demonstrated increased vascular endothelial growth factor and enhanced matrix metallopeptidase 2 (MMP-2) and -9 expression, along with endothelial dysfunction in animal models of DMD.36⇓-38
We did not observe any significant voxelwise correlation between any of the neuropsychological examination scores and WM alterations revealed by the DTI metrics. However, we did notice poor neuropsychological scores along with impaired WM integrity in the patients in the Dp140– subgroup. The lack of a correlation between WM changes and the extent of neuropsychological examination abnormalities needs to be resolved. Similarly, a lack of correlation between clinical and radiologic parameters was observed in the DTI study by Doorenweerd et al.6 However, another study did identify a significant correlation between clinical scores and DTI metrics of the corpus callosum.7
In the current study, most patients were receiving steroids at the time of evaluation. Steroids can potentially confound DTI results; a few studies in patients with Cushing disease have shown reduced integrity of the cerebral WM.39,40 However, no significant difference was noted in the duration or dose of steroids in the patients in the 2 subgroups, which suggests that the observed differences in the DTI metrics were most likely due to the primary disease and not steroid intake. Steroids partly alleviate intellectual impairment in patients with DMD41 as well as reverse BBB dysfunction and may increase the levels of shorter isoforms of dystrophin, as demonstrated in animal studies.42 Longitudinal studies are required to document the time course of the effects of steroidal treatment.
The limitations of the current study include the relatively low directional resolution of DTI. However, considering the pediatric population’s proneness to motion, a relatively shorter EPI sequence protocol like ours was considered robust. Accelerating acquisition using a multiband technique holds promise for the future to obtain high-resolution multishell imaging data for structural connectomics analyses and multicompartment modeling.
CONCLUSIONS
Children with DMD show widespread structural WM changes, which are more severe and widespread in children with distal mutations. Children with distal mutations have more severe abnormal findings in neuropsychological tests compared with the children with proximal mutations.
References
- Received December 9, 2019.
- Accepted after revision March 23, 2020.
- © 2020 by American Journal of Neuroradiology