Abstract
SUMMARY: The cerebral ventricles have been studied since the fourth century BC and were originally thought to harbor the soul and higher executive functions. During the infancy of neuroradiology, alterations to the ventricular shape and position on pneumoencephalography and ventriculography were signs of mass effect or volume loss. However, in the current era of high-resolution cross-sectional imaging, variation in ventricular anatomy is more easily detectable and its clinical significance is still being investigated. Interpreting radiologists must be aware of anatomic variations of the ventricular system to prevent mistaking normal variants for pathology. We will review of the anatomy and development of the lateral ventricles and discuss several ventricular variations.
The cerebral ventricles were the center of attention among philosophers, priests, anatomists, and physicians as far back as Aristotle in the fourth century BC.1 They were originally thought to harbor the soul and “vital” spirits responsible for higher functions. After the influence of Christianity and the Renaissance, the ventricles were conceptualized as 3 cavities where common sense, creative imagination, and memory were individually allocated. It was not until the 16th century that anatomists Andreas Vesalius and Constanzo Varolio identified ventricles as being filled with CSF.2
Before the era of cross-sectional imaging, when ventriculography and pneumoencephalography were the only modalities available, variations in ventricular morphology were used as signs of parenchymal volume loss or mass effect.3,4 Currently, asymmetries and anatomic variations are frequently identified and accepted as normal variations. Although they are not frequently a diagnostic dilemma, imaging interpreters must be aware of these anatomic variants to prevent mistaking them for pathology. In this review, we discuss normal anatomy, ventricular development, and anatomic variations of the lateral ventricles along with a deep look into the investigation of these entities. Pathologic states (ie, hydrocephalus, degenerative disease, traumatic encephalopathy, and so forth) can affect ventricular size and morphology but have been studied extensively and will be left out of this review.
ANATOMY
Lateral Ventricles
The lateral ventricles are paired C-shaped structures comprising a body and atrium along with 3 projections into the frontal, temporal, and occipital lobes, termed “horns.” The lateral ventricles communicate with the third ventricle through the interventricular foramina of Monro.5 Each lateral ventricle has an estimated capacity of 7–10 mL.6
The frontal horn (Fig 1) extends anteriorly from the foramina of Monro and communicates with the body of the lateral ventricles posteriorly. The anterior wall and roof are formed by the genu of the corpus callosum, and the floor is formed by the rostrum. The head of the caudate nucleus forms the lateral wall. The columns of the fornix form the inferior portion of the medial wall.6⇓-8
Anatomy of the frontal horns of the lateral ventricles. Coronal T1-weighted MR image from a healthy 22-year-old man. CN indicates caudate nucleus; CC, body of the corpus callosum; F, columns of the fornices; SP, septum pellucidum. Illustration adapted with permission from Gray.64
The body of the lateral ventricle communicates with the atrium posteriorly from the foramina of Monro to the corpus callosum and psalterium of the fornix, also called the hippocampal commissure. The roof is formed by the body of the corpus callosum, and the floor is formed by the thalamus. The septum pellucidum and body of the fornix form the superior and inferomedial walls, respectively. The lateral wall is formed by the caudate nucleus and thalamus. In the groove between the caudate nucleus and thalamus along the lateral wall sits the stria terminalis, a main outlet pathway of the amygdala and the location of the thalamostriate vein.9
The atrium (Fig 2) is a triangular cavity that communicates with the body, temporal horn, and occipital horn. The body and splenium of the corpus callosum form the roof. The tapetum, which is a sheetlike bundle of decussating fibers in the splenium of the corpus callosum, arches over the atrium and forms the roof. The tapetum continues laterally and comprises the lateral wall and the caudate nucleus. The floor is created by the collateral trigone, a continuation of the collateral eminence formed by the collateral sulcus. The contour of the medial wall is formed by the calcar avis and bulb of the corpus callosum, which is a bulging created by the forceps major.5,9 The calcar avis was previously known as the hippocampus minor and represents an indentation formed by the calcarine fissure.10 When prominent, the calcar avis can mimic hemorrhage on cranial sonography.11
Anatomy of the atrium of the left lateral ventricle. Coronal T1-weighted MR image from a healthy 21-year-old woman. OB indicates occipital bulb containing fibers of forceps major; CA, calcar avis; T, collateral trigone; CC, tapetum of the corpus callosum; solid arrow, calcarine sulcus; open arrow, collateral sulcus. Illustration adapted with permission from Gray.65
The temporal horn is the longest and largest horn, extending anteriorly from the atrium below the thalamus and terminating at the amygdala. The collateral eminence and hippocampus form the floor, which is separated from the hippocampus by a thin layer of white matter called the alveus.11,12 The roof is created by the thalamus, tail of the caudate nucleus, and tapetum. The striothalamic sulcus separates the caudate tail and thalamus. The tapetum also runs inferiorly to comprise the lateral wall and separates the lateral wall from the optic radiations. The choroidal fissure runs along the medial wall.12
The occipital horn curves posteriorly and medially from the atrium and varies in size. The tapetum forms the roof and lateral wall and separates it from the optic radiations.7,9,11 The collateral trigone forms the floor. Similar to the atrium, the bulb of the corpus callosum and calcar avis form the medial wall.9
Choroid Plexus and Choroidal Fissure
The choroid plexus is present within the lateral, third, and fourth ventricles and is the primary source of CSF, producing approximately 500 mL per day.13,14 Within the lateral ventricles, the choroid plexus runs along a cleft between the fornix and thalamus called the choroidal fissure. The choroidal fissure forms a C-shape extending from the foramina of Monro to its inferior terminal point, which is termed the “inferior choroidal point.”9,15 Within the atria, there is a prominent triangular tuft called the glomus.15 The tela choroidea is an invagination of the pia mater and ependyma, which gives rise to the choroid plexus within the choroidal fissure and along the roof of the third ventricle.9,16
The choroidal fissure is an important neurosurgical structure because it opens up access to the basal cisterns and to structures that would be otherwise inaccessible through an extracerebral route.9 Recently, excess CSF has been shown to pass through the inferior choroidal point in patients with idiopathic normal pressure hydrocephalus. Its anatomic relationship may explain disproportionate enlargement of the basal cisterns and Sylvian fissures in patients with idiopathic normal pressure hydrocephalus.17
DEVELOPMENT
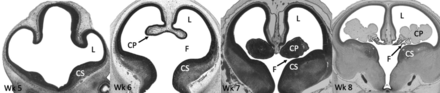
After closure of the neural tube during the fourth week of gestation, the lateral ventricles begin forming from the 2 outpouchings of the telencephalon, a derivative of the prosencephalon.18,19 The lateral walls of the telencephalon evaginate, and there is rapid growth of the medial basal portion of the cerebral hemispheres called the corpus striatum, which ultimately gives rise to the basal ganglia. This rapid growth shapes the floor and narrows the foramina of Monro (Fig 3).18,20⇓-22
Histologic sections of the telencephalon in the developing embryo from weeks 5 to 8. The lateral ventricles (L) arise as outpouchings from the telencephalon. Rapid growth of the corpus striatum (CS) shapes the floor of the lateral ventricles and narrows the foramina of Monro (F). The primitive choroid plexus (CP) comprises a large portion of the ventricular cavity by the eighth week. Also note that the lateral ventricles occupy a large majority of the cerebral hemisphere at 8 weeks. Images courtesy of Dr John Cork, PhD, Department of Cell Biology and Anatomy at Louisiana State University Health Sciences Center and the Virtual Human Embryo Project. Images used were the following: Carnegie Stage 17, section 410; Carnegie stage 18, section 302; Carnegie stage 20, section 131; Carnegie stage 23, section 50. https://www.ehd.org/virtual-human-embryo.
During the sixth week, the foramina of Monro begins to narrow (Fig 3), and the choroidal fissure forms.20,21 By the eighth week, further growth of the corpus striatum forms the C-shaped appearance of the lateral ventricles. The frontal and temporal horns are now well-defined, and the choroid plexus nearly fills the entirety of the lateral ventricles (Fig 3).21
At the end of the first trimester, the lateral ventricles continue to expand rapidly, occupying a large majority of the cerebral hemispheres, outpacing parenchymal growth.23 Transient occlusion of the central canal of the spinal cord may facilitate this rapid increase in ventricle size.24 Parenchymal growth hastens during the second trimester, and by 21 weeks, the frontal, temporal, and occipital horns are well-defined. By 31 weeks, the lateral ventricles resemble the adultlike appearance.25,26
ANATOMIC VARIANTS
Coarctation
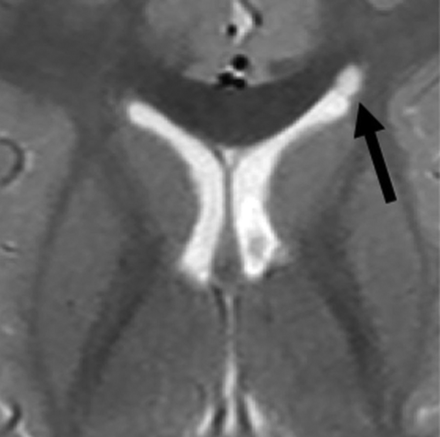
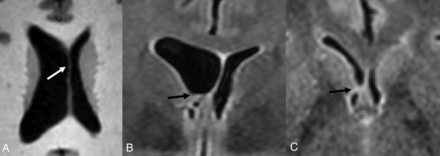
Coarctation (Fig 4) refers to the apposition or fusion of 2 ventricular walls, resulting in partial or complete obliteration of the lumen.27 When focal, coarctation can isolate a portion of the ventricle, creating multiple small rosettes or an ependymal-lined cyst, commonly referred to as a connatal cyst (Fig 4).28,29 The incidence of unilateral or bilateral frontal horn coarctation has been reported to be between 0.38% and 6.0%, with a majority of studies reporting incidences below 1%.30⇓-32 Coarctation of the occipital horns is much more common, with a reported incidence of 21.3%.33
Coarctation of the frontal horn. Axial T2-weighted MR image from a 3-year-old boy showing coarctation of the left frontal horn with connatal cyst formation (black arrow).
Coarctation is thought to occur at sites where opposing walls are in close proximity as is in the case with the ventricular horns, but it also has been described as occurring in the body.28,31,33 The exact mechanism of ventricular coarctation is currently unknown. Bates and Netsky28 described intermingling of subependymal glial cells at sites where the ventricular wall lacks ependyma. Davidoff27 reported similar findings in cases of congenital aqueductal stenosis.34 No histologic evidence of inflammation or gliosis has been associated with coarctation, suggesting that it is developmental in nature.27,28
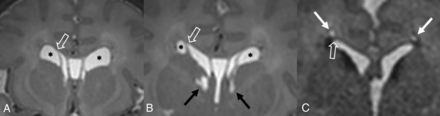
Ventricular coarctation is accepted as benign and is associated with normal neurodevelopmental outcomes when occurring in isolation.29,35 When identified in neonates, coarctation often resolves spontaneously by 2 months of age.36 However, it can commonly be mistaken for similar-appearing subependymal cysts or periventricular leukomalacia, which can be associated with less favorable outcomes.36⇓-38 Identifying the location of the cyst with respect to the superolateral angles of the lateral ventricles, which are formed by the most superior and lateral margins, is a key distinguishing feature. Connatal cysts (Fig 5A) occur anterior to the foramina of Monro and at the level of the angles. Periventricular leukomalacia (Fig 5B) should be considered when cyst formation is located above the angles, and subependymal cysts (Fig 5C) should be considered when it is located below the angles and at the caudothalamic groove.11,39,40
Periventricular cysts. A, Coronal T2-weighted MR image from a 1-week-old girl with a history of germinal matrix hemorrhage demonstrating coarctation of the bilateral frontal horns with connatal cyst formation (asterisks). Note that these cysts are at the level of the superolateral angles of the lateral ventricle (open arrow). B, Coronal T2-weighted MR image from the same patient demonstrating subependymal cysts (black arrows) located near the caudothalamic groove below the level of the superolateral angles (open arrow). Additional connatal cysts are present (asterisks). C, Coronal T2-weighted MR image from a 1-month-old boy with a history of germinal matrix hemorrhage and peripartum hypoxia demonstrating small bilateral periventricular cysts (white arrows) located above the level of the superolateral angles (open arrow). This appearance is consistent with cystic periventricular leukomalacia.
Asymmetric Size and Morphology
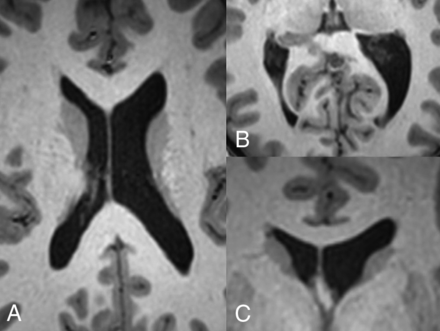
Size asymmetry between the lateral ventricles (Fig 6) occurs in 5%–12% of healthy individuals.41 In healthy patients, some studies have found that either the right or left lateral ventricle was consistently larger than the other,26,42⇓⇓⇓⇓-47 while other studies have reported no significant difference in size between the two.48,49 The association between lateral ventricle asymmetry, handedness, and sex is also controversial, with multiple studies showing a significant difference in lateral ventricle size in right- versus left-handed individuals and males versus females.45,49,50 Other studies have shown no significant difference in these populations.41,43,47,48
Variations in lateral ventricular size and shape in a healthy 16-year-old girl. Axial T1-weighted MR images through the bodies of the lateral ventricles (A) and occipital horns (B) demonstrate a larger left lateral ventricle. Note the differences in morphology of the occipital horns. C, Coronal T1-weighted MR image through the frontal horns demonstrates relative enlargement of the left lateral ventricle.
The shape of the lateral ventricles has also been investigated extensively, with multiple studies classifying various lateral ventricular morphologies and rotational differences.42,51⇓-53 The occipital horn of the lateral ventricle seems to be the most inconsistent portion, which can range from being completely absent to being present in variable lengths (Fig 6B).33,48,54 The temporal horn, especially the anterior tip, is also variable in shape but to a lesser extent compared with the occipital horn.55
The clinical significance of varying lateral ventricular asymmetries is currently controversial. On the basis of the association of ventricular morphologic abnormalities due to underlying white matter damage, it has been suggested that mild ventricular asymmetries may be a sign of subtle white matter or deep gray matter abnormalities that may not be seen on imaging. Paquette et al51 proposed that differences in the left lateral ventricle in preterm neonates may be related to subcortical white matter alterations, suggesting subcortical vulnerability to preterm birth.42 Sadan et al56 studied fetuses with normal-sized-but-asymmetric ventricles (defined as width of < 10 mm and a difference in width of > 2 mm) and demonstrated increased behavioral abnormalities in these children.
To our knowledge, no definitive large-scale investigation of variations in lateral ventricle morphology and long-term neurologic outcome has been performed. Therefore, it is generally accepted that small volumetric or morphologic differences between normal-sized lateral ventricles are likely of no clinical significance and have no effect on long-term neurodevelopmental outcome.3,41,44,48,52,57⇓-59 However, interpreting radiologists should evaluate adjacent parenchymal disease, an intraventricular lesion, or obstruction at the foramina of Monro before dismissing ventricular asymmetry as a anormal variant (Fig 7).60
Lateral ventricular asymmetry in a 56-year-old woman referred from a separate institution for the management of a suspected intraventricular mass. A, Axial T1-weighted MR images demonstrate asymmetric enlargement of the right lateral ventricle. There is mild bulging of the septum pellucidum (white arrow), which prompted careful evaluation of the lateral ventricular outflow. Coronal (B) and Axial (C) T2-weighted FLAIR images show no intraventricular mass but rather coaptation of the ependyma covering the caudate nucleus and fornix (black arrow). These imaging findings causes partial outflow obstruction and unilateral hydrocephalus. This remained stable on multiple subsequent follow-up MRIs and was favored to represent coarctation versus scar.
Cavum Septi Pellucidi, Cavum Vergae, and Cavum Veli Interpositi
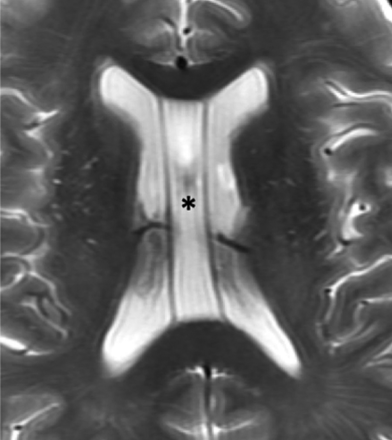
The septum pellucidum forms the medial walls of the lateral ventricles and consists of 2 thin laminae, which normally fuse shortly after birth. If the laminae fail to fuse, the potential space can expand with CSF, termed “cavum septi pellucidi.” If there is an extension posterior to a vertical plane created by the columns of the fornix, the term cavum vergae (Fig 8) is used.6,39 The clinical significance of cavum septi pellucidi and cavum vergae is unknown, and they are considered a normal variant.39 However, studies have suggested that their presence is associated with cognitive dysfunction in patients with prior head trauma.61 Also, the size and frequency of cavum septi pellucidi and cavum vergae were greater in patients with schizophrenia, alcoholism, and prior head trauma.62 The cavum septi pellucidi is a normal finding in fetuses between 18 and 37 weeks of gestation, and its absence is associated with multiple significant CNS abnormalities.63
Cavum septi pellucidi and vergae. Axial T2-weighted MR image of a 67-year-old man demonstrating expansion of the cavum septi, consistent with cavum septi pellucidi (asterisk). There is extension posterior to the vertical plane of the columns of the fornix (not shown here) consistent with coexisting cavum vergae.
The cavum veli interpositi results from expansion of the cistern of the velum interpositum, which is located above the third ventricle and below the columns of the fornices. When large, it may mimic a cyst. The cavum veli interpositi is accepted as a normal variant, and no association with cognitive dysfunction or neuropsychiatric disorders has been reported.39
Choroid Plexus Cysts
Choroid plexus cysts may be acquired or congenital. The acquired cysts are common in adults and are formed by accumulation of xanthogranulomatous debris; hence, these are more frequently referred to as xanthogranulomas. Xanthogranulomas are usually bilateral and demonstrate T1 and T2 signal characteristics similar to those of CSF but may not be completely suppressed on FLAIR. They are also typically hyperintense on DWI, with isointense signal on ADC (Fig 9A).5,6
Xanthrogranuloma and choroid plexus metastasis. Axial DWI (A) and postgadolinium T1-weighted (B) MR images of a 56-year-old woman with metastatic renal cell carcinoma. Images demonstrate a cystic lesion centered within the right choroid plexus glomus, which demonstrates increased DWI signal but no central enhancement (arrow), consistent with a xanthogranuloma. This finding is in contrast to the solidly-enhancing lesion centered within the left glomus, consistent with a hypervascular metastasis.
Choroid plexus cysts are typically found incidentally, mainly in the glomera, and they are usually asymptomatic. In the antenatal period, choroid plexus cysts may be associated with chromosomal abnormalities when large or when present with other CNS abnormalities.39
An important differential consideration is a choroidal metastasis, which may mimic xanthogranulomas, especially when cystic. However, the typical bilateral occurrence of xanthogranulomas and lack of internal enhancement can help differentiate the two (Fig 9B). An ependymal cyst is also a differential consideration, but these typically more closely follow CSF signal. They also arise outside the choroid plexus and displace the glomera, unlike xanthogranulomas.5
CONCLUSIONS
Isolated anatomic variations of the lateral ventricles are very common in healthy individuals. In this article, we describe several anatomic variants of the lateral ventricles and review multiple studies of these entities. We also review the anatomy and embryology of the lateral ventricles. These variations are often mentally noted while interpreting studies, and because they are generally accepted as normal and of no clinical significance to the patient, they are infrequently mentioned in radiology reports. Although identification of these is rarely a diagnostic dilemma, interpreting radiologists should be aware of these entities because some cases may mimic pathology.
Indicates open access to non-subscribers at www.ajnr.org
References
- Received November 12, 2019.
- Accepted after revision January 6, 2020.
- © 2020 by American Journal of Neuroradiology