Abstract
BACKGROUND AND PURPOSE: In glioblastoma, tumor-associated macrophages have tumor-promoting properties. This study determined whether routine MR imaging features could predict molecular subtypes of glioblastoma that differ in the content of tumor-associated macrophages.
MATERIALS AND METHODS: Seven internally derived MR imaging features were assessed in 180 patients, and 25 features from the Visually AcceSAble Rembrandt Images feature set were assessed in 164 patients. Glioblastomas were divided into subtypes based on the telomere maintenance mechanism: alternative lengthening of telomeres positive (ALT+) and negative (ALT−) and the content of tumor-associated macrophages (with [M+] or without [M−] a high content of macrophages). The 3 most frequent subtypes (ALT+/M−, ALT−/M+, and ALT−/M−) were correlated with MR imaging features and clinical parameters. The fourth group (ALT+/M+) did not have enough cases for correlation with MR imaging features.
RESULTS: Tumors with a regular margin and those lacking a fungating margin, an expansive T1/FLAIR ratio, and reduced ependymal extension were more frequent in the subgroup of ALT+/M− (P < .05). Radiologic necrosis, lack of cystic component (by both criteria), and extensive peritumoral edema were more frequent in ALT−/M+ tumors (P < .05). Multivariate testing with a Cox regression analysis found the cystic imaging feature was additive to tumor subtype, and O6-methylguanine methyltransferase (MGMT) status to predict improved patient survival (P < .05).
CONCLUSIONS: Glioblastomas with tumor-associated macrophages are associated with routine MR imaging features consistent with these tumors being more aggressive. Inclusion of cystic change with molecular subtypes and MGMT status provided a better estimate of survival.
ABBREVIATIONS:
- ALT
- alternative lengthening of telomeres
- ALT−
- alternative lengthening of telomeres negative
- ALT+
- alternative lengthening of telomeres positive
- IDH
- isocitrate dehydrogenase
- M+
- a tumor with a high content of tumor-associated macrophages
- M−
- a tumor with a low content of tumor-associated macrophages
- MGMT
- O6-methylguanine methyltransferase
- PTE
- peritumoral edema
- VASARI
- Visually AcceSAble Rembrandt Images
Glioblastomas are the most common and aggressive primary malignant brain tumor, with an incidence of 5.26 per 100,000 population.1 With temozolomide and radiation therapy, the median survival is 14.6 months.1 Patients' response to treatment and prognosis are notoriously variable. Many studies have attributed heterogeneous outcomes to molecular differences among glioblastomas, such as different means to maintain telomere integrity (telomerase activity or an alternative method known as the alternative lengthening of telomeres [ALT]).2 In 2003, the presence of the ALT telomere maintenance mechanism was associated with longer patient survival compared with ALT negative (ALT−) tumors, including those that were telomerase positive (ALT+) and those where the telomere maintenance mechanism was unknown.3
It has been recently found that most tumors without a defined telomere maintenance mechanism contain a high content of tumor-associated macrophages (M+) that affects assays assigning telomere maintenance.4 The revised subgroups were ALT positive tumors with and without (M−) a high content of macrophages (ALT+/M+ and ALT+/M−) and ALT negative tumors with and without a high content of macrophages (ALT−/M+ and ALT−/M−). With temozolomide treatment, patients with ALT−/M− tumors have improved survival compared with patients with ALT−/M+ tumors,4 suggesting that identifying the increased macrophage content will distinguish those with a poor outcome.
MR imaging is a diagnostic tool that evaluates tumor as well as peritumoral characteristics. Improving prognostic determination based on MR imaging features alone has associated radiologic necrosis and peritumoral edema (PTE) with poorer survival.5⇓⇓–8 Tumors with a cystic component have been associated with improved patient survival.9,10 However, inconsistencies occur between studies, and MR imaging features are not always found as independent factors associated with survival.11⇓⇓–14
Imaging features have been attributed to differences in tumor biology, suggesting an analysis of MR imaging features combined with molecular characteristics may improve prognostic prediction. Isocitrate dehydrogenase 1 (IDH1) mutations, O6-methylguanine methyltransferase (MGMT) promoter methylation, and epidermal growth factor receptor overexpressing tumors have been correlated with MR imaging features.13,15⇓⇓⇓–19 That tumor biology influences imaging features is further evident in studies that investigate subgroups of glioblastomas based on multiple gene expression differences.20
This study aimed to determine whether MR imaging features were associated with the telomere maintenance mechanism and tumor-associated macrophage content–based subtypes. The inclusion of MR imaging parameters with the molecular subtyping and MGMT promoter methylation status21 was also investigated to determine whether it could better predict outcome for patients with glioblastoma.
Materials and Methods
Study Participants
One hundred eighty patients from Dunedin, Christchurch, and Waikato hospitals in New Zealand with a diagnosis of glioblastoma between the years 2004 and 2014 were included in the study. The cohort was aged between 16–82 years (mean, 61 years; 95% CI, 59–63); 39% were female and 61% male; and 93% were white, 3% Maori, and 4% identified with other ethnic groups. All patients were eligible for standard of care therapy (surgery, radiation therapy, and temozolomide), and no patients received other therapies. The participants were all diagnosed with glioblastoma after the Stupp protocol was used in New Zealand.1
Participant Selection/Inclusion Criteria
The inclusion criteria were a diagnosis of glioblastoma (as assessed by 2 pathologists independently), no previous lower-grade brain tumor or other brain surgery, and no previous radiation or chemotherapy. No patients received corticosteroids at the time of the preoperative MR imaging scan. Survival time was defined as the time interval (months) between the time of diagnosis (defined as the time of the initial preoperative MR imaging scan) and death.
MR Imaging Features and Interpretation
Preoperative MR imaging scans were evaluated including T1, T2, FLAIR, and postcontrast T1-weighted sequences after intravenous infusion of 10 mL of gadolinium. Images were taken by clinical 1.5T (Siemens, Erlangen, Germany or GE Healthcare, Milwaukee, Wisconsin) scanners. All study scans had routine tumor protocol sequences (T1, T2, FLAIR, DWI, ADC, and postcontrast gadolinium T1), and some had additional susceptibility-weighted sequences. At least 3 reviewers (3 neuroradiologists [M.V.R., B.K.J.W., and D.A.B.] and 1 radiology registrar [J.Z.]) read each MR imaging scan independently in the Public Hospital PACS. All readers were blinded to patient demographics, treatment regimen, and tumor molecular subtypes. The term “overall agreement” was defined as when 3 or more examiners agreed and the term “overall disagreement” was defined as when 2 or more examiners disagreed. In cases with disagreement between reviewers, the cases were reanalyzed in a collaborative fashion by at least 3 examiners, and a consensus score was reached and used in the final analysis.
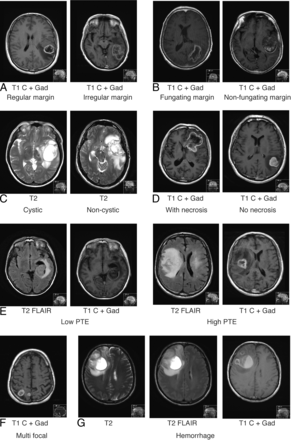
Based on the current available MR imaging literature and the cumulative experiences from the 2 tertiary centers, 7 imaging features were analyzed, as detailed in Table 1. These features included tumor margin: regular or irregular (Fig 1A, regular margin being a tumor with a smooth enhancing margin on postgadolinium T1 and irregular margin being a tumor that lacks a smooth enhancing margin on postgadolinium T1), fungating or nonfungating (Fig 1B, a fungating margin being a tumor with a thick heterogeneous “brush”-like enhancing rim and nonfungating margin being a tumor that lacks a thick heterogeneous “brush”-like enhancing rim), cystic component (Fig 1C), radiologic necrosis (Fig 1D), limited or extensive PTE (Fig 1E), multifocality (Fig 1F), and hemorrhage (Fig 1G).
Specific classification of MRI features
Examples of MR imaging features in glioblastoma. A, Tumor classified with a regular (left) or irregular margin (right). B, Tumor classified with a fungating (left) or nonfungating margin (right). C, Tumors classified with a cystic component (left) or noncystic component (right). D, Tumor classified with (left) and without (right) radiologic necrosis. E, Tumor classified with low (limited, left) and high (extensive, right) PTE. F, Tumor classified as being multifocal. G, tumor classified with hemorrhage. Gd indicated gadolinium; C, contrast.
The protocol for measuring PTE grade was based on that developed by Wu5 and Hartmann.22 The degree of the white matter edema was estimated on the basis of the maximal distance from the tumor margin to the furthest point of the white matter edema. The tumor's maximal dimension was estimated based on the maximal diameter of the tumor on any axis. When the degree of white matter edema was less or similar (no more than 4 mm greater) to the tumor's maximal dimension, the edema was considered low grade, and when the degree of white matter edema was greater (more than 4 mm) than the tumor's maximal dimension, the edema was considered a high-grade.
Furthermore, the Visually AcceSAble Rembrandt Images (VASARI) feature set was analyzed on 164 cases (https://wiki.cancerimagingarchive.net/display/Public/VASARI+Research+Project18,23,24); 16 cases were missing some sequences required for the VASARI analysis.
Telomere Maintenance Mechanism Subtyping
Tumors were classified into 4 subgroups: ALT positive tumors with or without a high content of tumor associated macrophages (ALT+/M+ and ALT+/M−, respectively) and ALT negative tumors with or without a high content of tumor associated macrophages (ALT−/M+ and ALT−/M−, respectively) based on established methods by using paraffin-embedded tumor sections.4,25 Tumors from 80 cases were typed for telomere maintenance mechanisms as part of an earlier study, and 100 tumors were new to this study.4 The tumor-associated macrophage content was identified by immunohistochemistry staining for CD163 positive macrophages. The CD163 antibody used was EPR19518 (Abcam, Cambridge, United Kingdom), and the macrophage content was estimated as described previously.4
Mutant IDH1 and IDH2 Determination
The presence of the R132H IDH1 mutation was estimated by using immunohistochemistry.26 To confirm the presence and frequency of IDH1 mutations and to estimate the number of IDH2 mutations, exon 4 of IDH1 and IDH2 were amplified from tumor-extracted DNA and sequenced.27 Tumor DNA was extracted from paraffin embedded tumor sections or frozen tumor.
MGMT Promoter Methylation Determination
The method to determine whether tumors had a methylated or unmethylated MGMT promoter was based on published methods.21,28 In this study, genomic DNA was extracted from paraffin-embedded tumors by using the QIAmp DNA FFPE tissue Kit (QIAGEN, Tokyo, Japan), and bisulfite DNA conversion was performed by using the EpiTect Bisulfite kit (QIAGEN).
Statistical Methods
The clinico-demographic and MR imaging features were compared between the 3 telomere maintenance–based subtypes by using Pearson χ2 tests and 1-way ANOVA as appropriate. The κ statistical test was performed to assess the concordance between interobserver agreement. Logistic regression was used to test if imaging could predict the ALT+/M− and ALT−/M+ subtypes and used imaging features as covariants. Survival was compared between the subtypes by using the log-rank test and the independent association of the MR imaging features in addition to the subtype effects. To test multiple variants on survival, Cox proportional hazards regression models were used. A 2-tailed P value <.05 was taken to indicate statistical significance.
Results
Whether the 7 internally derived MR imaging features could be consistently identified was investigated by comparing MR imaging scores from the 3 different readers and calculating the percentage of scans on which all 3 blinded reviewers agreed on the presence or absence of each MR imaging feature. κ statistical analyses were performed. The interobserver data including κ value demonstrated reproducible results (Table 2). The interobserver agreement rate was highest for the glioblastoma cystic component feature (86.2%, κ = 0.71), followed by multifocal lesion (85.6%, κ = 0.71). The margin-based parameters had the lowest interobserver agreement percentages (regular margin, 62.9%, κ = 0.23; fungating margin, 64.6%, κ = 0.28).
Interobserver reliability assessment for MRI features in glioblastoma
MR Imaging Features Predictive of ALT+ and M+ Status
In the 7 internally derived MR imaging feature groups, the clinico-demographic features and the frequency of IDH1 and IDH2 mutations and MGMT promoter methylation among the telomere maintenance subtypes are given in Table 3. Three telomere maintenance–based subtypes had sufficient numbers to investigate MR imaging features (ALT+/M−, n = 26; ALT−/M−, n = 92; and ALT−/M+, n = 56). Because of the lack of glioblastomas in the ALT+/M+ group (n = 6), these cases were not included in the subsequent analyses. The frequency of each MR imaging feature among the telomere-based subtypes is given in Table 4. Five MR imaging features—regular margin, fungating margin, tumor cystic component, radiologic necrosis, and limited or extensive PTE—showed altered frequency among the 3 tumor subgroups.
Clinico-demographic features among telomere maintenance glioblastoma subtypes
MRI features among telomere maintenance glioblastoma subtypes
The ALT−/M+ subgroup had the lowest incidence of tumors with a cystic component (10.7%) compared with the ALT+/M− (38.5%) and the ALT−/M− (23.9%) groups (P = .014). This subtype had the highest incidence of tumors with extensive PTE (44.6% versus 23.1% for ALT+/M− and 22.8% for ALT−/M−, P = .014) and the highest incidence of tumors with radiologic necrosis (85.7% versus 72.8% for ALT−/M− and 58% for ALT+/M−; P = .021).
The ALT+/M− subgroup had the highest incidence of tumors with a regular margin (58% versus 30% in both the ALT−/M− and ALT−/M+ groups; P = .026) and the lowest incidence of tumors with a fungating margin (19% versus 53% and 61% in the ALT−/M− and ALT−/M+ groups, respectively; P = .002).
With the exception of PTE, the other MR imaging features were strongly associated with each other. Cystic tumors were more likely to have regular margins with no necrosis, and noncystic tumors were more likely to have irregular margins with necrosis (cystic association with regular margin, P < .0001; cystic with no necrosis, P < .0001).
To test whether imaging could predict ALT+/M− or an ALT−/M+ status, a logistic regression analysis was performed. For predicting ALT+/M− tumors, 3 features—cystic component (standard error, 1.18; P = .0077; OR, 0.04; 95% CI, 0.004–0.43), radiologic necrosis (standard error, 1.16; P = .0001; OR, 84; 95% CI, 8.6–821), and fungating margin (standard error, 0.61; P = .015; OR, 0.23; 95% CI, 0.07–0.75) showed statistical differences and were included in the final model. For predicting ALT−/M+ tumors, 2 features—extensive PTE (standard error, 0.48; P = .007; OR, 0.27; 95% CI, 0.11–0.7) and fungating margin (standard error, 0.36; P = .014; OR, 2.5; 95% CI, 1.2–4.8) showed statistical differences and were included in the final model.
Using the VARSARI feature set, 6 VASARI features—side of lesion center (F2), proportion of necrosis (F7), cysts (F8), T1/FLAIR ratio (F10), proportion of edema (F14), and ependymal extension (F19)—were found to be correlated with the 3 tumor subgroups. The full set of VASARI features and the associations with telomere maintenance glioblastoma subtypes is detailed in On-line Table 1.
The ALT−/M+ subgroup had the lowest incidence of tumors with a cyst (4%) compared with the ALT+/M− (30%) and the ALT−/M− (12%) groups (P = .007), the highest incidence of tumors with a higher proportion of necrosis (71%) versus 49% for ALT−/M− and 42% for ALT+/M (P = .026), and the highest incidence of tumors with the highest grade of edema (grade 5; 53%) compared with 32% in the ALT−/M− and 14% in the ALT+/M− group (P = .002).
The ALT+/M− subgroup had the highest incidence of tumors with an expansive T1/FLAIR ratio (79%) compared with 41% in the ALT−/M− and 37% in the ALT−/M+ group (P = .0003), the lowest incidence of tumors located in the right side of the brain (17%) compared with 41% in the ALT−/M− and 54% in the ALT−/M+ group (P = .007), and the lowest incidence of tumors with ependymal extension (30%) compared with 60% in the ALT−/M− and 49% in the ALT−/M+ group (P = .036).
To test whether the VASARI imaging features could predict ALT+/M− or an ALT−/M+ status, a logistic regression analysis was performed. For predicting ALT+/M− tumors, 3 features were significant—F2 side of lesion center % left (standard error, 0.52; P = .049; OR, 2.8; 95% CI, 1.01–7.8), F8 cysts (standard error, 0.57; P = .04; OR, 3.2, 95% CI, 1.05–9.8), and F10 T1/FLAIR ratio % expansive (standard error, 0.52; P = .016; OR, 3.5; 95% CI, 1.26–9.7). To predict ALT−/M+ status, only 1 feature was significant: F8 cysts (standard error, 0.77; P = .03; OR, 0.19; 95% CI, 0.04–0.87).
MR Imaging Features Correlated with IDH1 and IDH2 Mutations
In the 7 internally derived MR imaging feature groups, the mutant IDH1 or mutant IDH2 was present in 30 tumors; this included most ALT+/M− (n = 19/26, 73%; Table 3) tumors and was less frequent in ALT−/M− (n = 8/92, 9%) and ALT−/M+ tumors (n = 3/56, 5%).
Tumors with mutant IDH1 and IDH2 had a higher incidence of the cystic component feature (37%) compared with tumors with wild-type IDH1 and IDH2 (17%, P = .017) and fewer incidences of tumors with a fungating margin (33%) compared with wild-type tumors (55%, P = .028; Table 5). Using the VASARI features, patients with mutant IDH1 and IDH2 glioblastomas had a higher incidence of tumors with an expansive T1/FLAIR ratio (80%) compared with the wild-type tumors (40%, P < .0001) and a lower incidence of grade 5 edema (17%) compared with wild-type tumors (38%, P = .024; On-line Table 2).
MRI features among mutant IDH1 and IDH2 tumors
MR Imaging Features Predict Survival Beyond Molecular Status
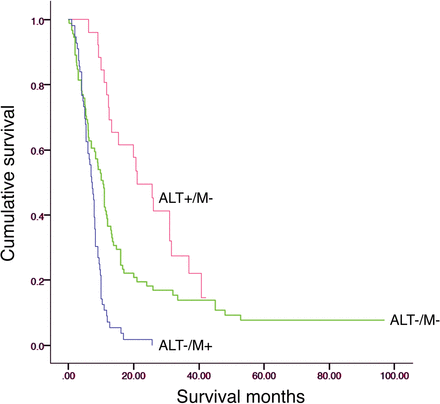
Consistent with earlier studies, the ALT+/M− subgroup patients had the best survival (Fig 2), and the ALT−/M− subgroup patients had an improved overall survival compared with the ALT−/M+ group (Fig 2, P < .009 ALT+/M− versus ALT−/M−, P < .0001 ALT+/M− versus ALT−/M+, and ALT−/M− versus ALT−/M+ P < .001).3,4,29 There were no significant differences (P > .05) among telomere maintenance–based subgroups in relation to the amount of patients treated with radiation therapy, concurrent temozolomide, or adjuvant temozolomide. In an earlier study and in this study (Table 4), the ALT−/M+ subgroup was associated with an increased frequency of partial resections compared with near-total resections.4
The 3 telomere maintenance mechanism subtypes are associated with differences in patient survival. Eighty cases were those used in a previous study,4 and 100 cases were new to this study.
A Cox regression analysis by using forward and backward stepwise models was used to determine whether 4 imaging features (margin regularity, cystic, necrosis, and PTE grade) added to telomere maintenance–based subtype and MGMT promoter methylation status to better determine patient prognosis. Cystic feature was significant (P = .045) in predicting patients' survival independent of tumor subtype and MGMT promoter methylation status (Table 6).
Cox regression analysis to test if radiology features added to telomere maintenance subtype and MGMT promoter methylation status in predicting patient survival
Discussion
An assessment of MR imaging features is an advantage to using histologic-based subtypes alone because the characteristics of whole tumor in situ can be assessed before surgery. In this study, we found radiologic features (including the VASARI MR imaging) associated with telomere maintenance and tumor-associated macrophage content–based subtypes, consistent with MR imaging features being informative in identifying differences in tumor biology. A cystic component was additive in predicting an improved patient outcome, suggesting that inclusion of this MR imaging feature along with an assessment of telomere maintenance subtypes and MGMT promoter methylation status will improve patient outcome prediction. Both feature sets had imaging parameters that predicted the ALT+/M− and the ALT−/M+ subgroups.
Because temozolomide use ALT−/M+ tumors are associated with the poorest survival independent of patient age, extent of surgery, and treatment received.4 ALT−/M+ tumors were associated with a higher proportion of necrosis, extensive edema, and lack of a cystic component. Whether the increased macrophage content directly contributes to the associated MR imaging features is unknown. Tumor-associated macrophages have many tumor-promoting activities including proinvasive and proangiogenesis properties.30 Increased edema may result from increased vascular permeability. Increased vascular endothelial growth factor and angiogenesis correlated with edema,31,32 and a molecular marker found on endothelial cells (deltalike ligand 4) that functions in angiogenesis was associated with PTE and poorer patient survival.33 Consistent with a link between PTE and angiogenesis, patients treated with the vascular endothelial growth factor inhibitor bevacizumab had reduced PTE.34
Increased edema has been associated with poorer prognosis.6⇓–8 In the study by Carillo et al,13 edema was additive for survival for MGMT methylated but not unmethylated tumors. In the current study, edema was not additive to survival by tumor subtype; this is likely attributable to edema being closely associated with the ALT−/M+ subtype. In a future study, we aim to incorporate quantitative MR imaging techniques, more specifically DTI in vivo to demonstrate the PTE grade.35 The imaging features associated with the ALT−/M+ group are consistent with this subgroup comprising aggressive tumors. The increased content of macrophages in the ALT−/M+ subtype, combined with these tumors having a poorer prognosis that has not changed with temozolomide, suggests this subtype would be an ideal target for immunotherapies, particularly those that could activate macrophages to remove the malignant cells or those that inhibit the tumor-promoting activities of tumor-associated macrophages.4,36
Alternative lengthening of telomere-positive tumors is associated with the best overall patient survival independent of patient age, extent of surgery, treatment received, and IDH1 mutant status.3,4,29 In this study, ALT+ tumors were more likely to have a regular margin, lack of a fungating margin, cystic component, expansive T1/FLAIR ratio, and lack of ependymal extension, consistent with ALT+ tumors being less aggressive. The MR imaging data from other studies support ALT+ tumors being less aggressive.29,37,38 Sharper margins and less contrast enhancement by MR imaging have been associated with features of ALT+ tumors, including mutant IDH1, mutant tumor protein p53 (TP53), or proneural tumors.13,16,18,20,39,40 Considering that most ALT+tumors in this study had mutant IDH1 or IDH2, MR imaging features of ALT may be due to mutant IDH. The expansive T1/FLAIR VASARI feature had greater significance when tumors were compared based on IDH mutant status rather than telomere maintenance subtype.
The presence of the cystic component was associated with improved survival. A cystic component has been associated with improved survival in some,9,10 but not all studies.6,12,13 If molecular differences are driving phenotypes that are detected by MR imaging, inconsistencies between studies may be improved if the molecular subgroups are included in analyses of MR imaging features. Tumors that were ALT+/M− and ALT−/M− had a higher cystic component compared with non-ALT+/M+ tumors. In a study by Carrillo et al,13 a cystic component was one of 4 features that could predict mutant IDH1 glioma with 94% accuracy. A cystic component associated with mutant IDH1 could explain the higher incidence of the cystic component in ALT+ tumors. Similarities exist between clinical parameters for those with cystic glioblastoma and those with secondary glioblastoma, suggesting the cystic component may indicate those with previously undiagnosed low-grade glioma. This may be the case for those with ALT+ tumors because ALT+/M− is more frequent in secondary glioblastoma. A cystic component was present in a considerable portion of ALT−/M− tumors; fewer of these had mutant IDH1 (n = 3/22, 13%), and most are thought to arise de novo.
The cystic component in ALT−/M− tumors may arise because of a different mechanism to that in ALT+ tumors. Why ALT−/M− tumors with a cystic component are associated with improved survival is unclear. The cystic component may represent a distinct tumor biology that responds well to treatment, or it may directly aid in the acquisition or detainment of temozolomide. The cystic component was reproducibly identified among different radiologists. A larger study to investigate whether ALT−/M− tumors with a cystic component are those that respond well to temozolomide is warranted.
Limitations of this study include its retrospective nature. The study excluded patients previously treated with radiation therapy to prevent factors that would affect MR imaging interpretation. This would exclude more patients with secondary glioblastoma that are more likely to be ALT+. Thus, the MR imaging features of the ALT+ group will be representative of a subset of ALT+ tumors.
Conclusions
The radiologic feature of cystic component was a predictive factor for survival and could be combined with telomere maintenance mechanism and macrophage content–based subtypes and MGMT promoter methylation status to provide a better estimation of survival. A lack of cystic component, a lack of left-sided tumor epicenter, higher necrosis and edema, along with a higher ependymal extension are more often present in tumors with a high tumor-associated macrophage content, suggesting that the presence of macrophages leads to a more invasive tumor behavior and a greater difficulty in obtaining near total resection, which could be predicted by MR imaging.
Acknowledgments
We would like to acknowledge Dr James Fulton for his MR imaging expertise, Mrs Amanda Fisher, Ms Sara Bowie, and Ms Janine Neill for their technical assistance, Ms Helen Morrin and the Cancer Society Tissue Bank, and Mr David Wong for his contribution to data collection.
Footnotes
Disclosures: Jean Zhou—RELATED: Grant: Health Research Council of New Zealand, Comments: titled “Molecular subtypes of glioblastoma predicts tumour behavior”*; UNRELATED: Employment: Southern District Health Board, Comments: Radiology registrar in Dunedin Hospital, Otago, New Zealand; Grants/Grants Pending: Health Research Council, Comments: grant will be used to work on glioblastoma subtyping and imaging clinical trial with ultrasmall superparamagnetic iron oxide particles*. Michael V. Reddy—UNRELATED: Board Membership: Pacific Radiology Group, Comments: a private diagnostic radiology practice; Employment: Pacific Radiology Group and Southern District health Board, Comments: public and private radiology groups for whom I provide radiology services. Ramona A. Eiholzer—RELATED: Grant: Health Research Council of New Zealand*. Tania Slatter—RELATED: Grant: Health Research Council New Zealand*. *Money paid to the institution.
This work was supported by the Health Research Council of New Zealand.
Paper previously presented at: American Roentgen Ray Society Annual Meeting, April 17–26, 2016; Los Angeles, California.
Indicates open access to non-subscribers at www.ajnr.org
References
- Received March 20, 2017.
- Accepted after revision September 7, 2017.
- © 2018 by American Journal of Neuroradiology