Abstract
BACKGROUND AND PURPOSE: The presence of apolipoprotein E4 (APOE*E4) is the strongest currently known genetic risk factor for Alzheimer disease and is associated with brain gray matter loss, notably in areas involved in Alzheimer disease pathology. Our objective was to assess the effect of APOE*E4 on brain structures in healthy elderly controls who subsequently developed subtle cognitive decline.
MATERIALS AND METHODS: This prospective study included 382 community-dwelling elderly controls. At baseline, participants underwent MR imaging at 3T, extensive neuropsychological testing, and genotyping. After neuropsychological follow-up at 18 months, participants were classified into cognitively stable controls and cognitively deteriorating controls. Data analysis included whole-brain voxel-based morphometry and ROI analysis of GM.
RESULTS: APOE*E4-related GM loss at baseline was found only in the cognitively deteriorating controls in the posterior cingulate cortex. There was no APOE*E4-related effect in the hippocampus, mesial temporal lobe, or brain areas not involved in Alzheimer disease pathology. Controls in the cognitively deteriorating group had slightly lower GM concentration in the hippocampus at baseline. Higher GM densities in the hippocampus, middle temporal lobe, and amygdala were associated with a decreased risk for cognitively deteriorating group status at follow-up.
CONCLUSIONS: APOE*E4-related GM loss in the posterior cingulate cortex (an area involved in Alzheimer disease pathology) was found only in those elderly controls who subsequently developed subtle cognitive decline but not in cognitively stable controls. This finding might explain the partially conflicting results of previous studies that typically did not include detailed neuropsychological assessment and follow-up. Most important, APOE*E4 status had no impact on GM density in areas affected early by neurofibrillary tangle formation such as the hippocampus and mesial temporal lobe.
ABBREVIATIONS:
- AD
- Alzheimer disease
- APOE
- apolipoprotein E
- dCON
- cognitively deteriorating controls
- MCI
- mild cognitive impairment
- sCON
- cognitively stable controls
The apolipoprotein E gene (APOE) has 3 different alleles, APOE*E2, APOE*E3, and APOE*E4. The presence of APOE*E4 is the strongest currently known genetic risk factor for Alzheimer disease (AD).1,2 APOE*E3 is the most common variant in the general population, while the APOE*E2 variant is associated with a lower risk of AD. The APOE variant modifies not only the risk of AD but also the age of onset of cognitive symptoms.3,4
Cross-sectional structural MR imaging studies indicated reduced gray matter in elderly APOE*E4 carriers including healthy controls, subjective memory impairment, mild cognitive impairment (MCI), and AD.5⇓⇓⇓⇓⇓⇓⇓⇓–14 In MCI and AD, the APOE*E4-related GM decrease seems to affect areas involved in AD pathology, notably the hippocampus, amygdala, and mesial temporal cortex14⇓⇓–17 but also the left occipital, frontal, and anterior cingulate cortices.14,18 In healthy elderly controls, the APOE*E4 effect on brain structure is less clear. Decreased GM volume in the caudate nuclei14 and the right cingulate gyrus and decreased white matter integrity in right parahippocampal gyrus19 were found. However, negative data were also reported.15,18,20 In younger APOE*E4 carriers, the results are also more ambiguous. Some studies demonstrated reduced GM in middle-aged21 and young APOE*E4 carriers22,23 compared with the other APOE allele carriers, whereas others reported no APOE*E4 effect throughout adulthood.20
Longitudinal studies also indicated that among MCI converters, those with a positive APOE*E4 status displayed increased GM atrophy in AD-related brain regions.24,25
The current investigation goes 1 step earlier in the degenerative process and assesses the effect of APOE allele status in healthy controls who subsequently developed subtle cognitive decline. To this end, we performed MR imaging and cognitive assessment at baseline in 382 community-dwelling elderly controls. Extensive cognitive assessment was repeated at 18-month follow-up to define a subsample of 181 individuals with a stable condition and 201 with a deteriorating condition. We demonstrated a gradually progressive GM loss in the posterior cingulate cortex as a function of APOE alleles (E2 < E3 < E4) only in deteriorating groups, with preserved GM densities in cognitively stable groups.
Materials and Methods
Study Protocol and Participants
All data used in the preparation of this article were obtained from a large, population-based study of community-dwelling older adults who have undergone an 18-month follow-up. These community-dwelling subjects were recruited via advertisements in local newspapers and media. All participants had normal or corrected-to-normal visual acuity, and none reported a history of neurologic or psychiatric disorders or alcohol or drug abuse. To avoid contamination by reversible forms of cognitive decline, subjects with vitamin B12 or folic acid deficiency and those with infectious diseases were excluded. Subjects with regular use of neuroleptics, antidepressants, mood stabilizers, anticonvulsant drugs, or psychostimulants and those with contraindications to MR imaging were excluded. Initially, 433 patients were screened. Thirty-seven refused to continue after the first evaluation (no death or illness, but for personal reasons). Fourteen were not considered due to the above-mentioned exclusion criteria. Five among them had substantial abnormal findings on MR imaging at baseline.
The education level was defined according to the Swiss scholar system, in which level 1 = <9 years (primary school), level 2 = between 9 and 12 years (high school), and level 3 = >12 years (university). All participants provided written informed consent after formal approval by the local ethics committee.
Neuropsychological Assessment
At baseline, all individuals were evaluated with an extensive neuropsychological battery, including the Mini-Mental State Examination,26 the Hospital Anxiety and Depression Scale,27 and the Lawton Instrumental Activities of Daily Living.28 Cognitive assessment included the following: 1) attention (Digit-Symbol-Coding,29 Trail-Making Test A,30); 2) working memory (verbal: Digit Span Forward31; visuospatial: visual memory span [Corsi]32); 3) episodic memory (verbal: RI-48 Cued Recall Test33; visual: Shapes Test34); 4) executive functions (Trail-Making Test B,30 Wisconsin Card Sorting Test, and Phonemic Verbal Fluency Test); 5) language (Boston Naming Test35); 6) visual gnosis (Ghent Overlapping Figures); and 7) apraxia: ideomotor,36 reflexive,37 and constructional (Consortium to Establish a Registry for Alzheimer Disease; figure copy test38). All individuals were also evaluated with the Clinical Dementia Rating scale.39
Those who met dementia the Diagnostic and Statistical Manual of Mental Disorders, 4th edition, diagnostic criteria based on the neuropsychological and clinical assessments were excluded. In agreement with the criteria of Petersen et al,40 participants with a Clinical Dementia Rating scale score of 0.5 but no dementia and a score of >1.5 SDs below the age-appropriate mean in any of the previously mentioned tests were also excluded from the present study. Participants with neither dementia nor MCI were classified as cognitively healthy older controls and underwent full neuropsychological assessment at follow-up by the same neuropsychologist, on average 18 months later. Those whose cognitive scores remained stable and those whose performance at follow-up was at least 0.5 SD lower compared with the baseline evaluation in at least 2 cognitive tests were classified as stable (sCON) and deteriorating (dCON), respectively. Two neuropsychologists clinically assessed all individuals independently. The final classification of sCON versus dCON was made by a trained neuropsychologist who took into account both the neuropsychological test results and overall clinical assessment.41 In her final appreciation, the trained neuropsychologist considered the most relevant test performances, usually altered in the course of Alzheimer disease (verbal episodic memory: RI-48 Cued Recall Test; attention: Trail-Making Test A; executive functions: Trail-Making Test B30 and Phonemic Verbal Fluency Test). Among the 2 tests needed for dCON classification, altered cognitive scores in at least 1 of these tests were mandatory.
MR Imaging Acquisition
Imaging data were acquired on a clinical routine whole-body 3T MR scanner (Tim Trio; Siemens, Erlangen, Germany). The structural 3D T1 sequence was performed with the following fundamental parameters: 256 × 256 matrix; 176 sections; 1 × 1 × 1 mm3; TE, 2.3 ms; TR, 2300 ms. Additional sequences (T2WI, susceptibility-weighted imaging, diffusion tensor imaging) were used to exclude incidental brain lesions.
Genetic Testing
Whole-blood samples were collected at baseline for all subjects for APOE genotyping. Standard DNA extraction was performed by using either 9-mL ethylenediaminetetraacetic acid tubes (Sarstedt AG, Nümbrecht, Germany) or the Oragene Saliva DNA Kit (DNA Genotek; Kanata, Ontario, Canada); tubes were stored at −20°C. APOE genotyping was performed on the LightCycler (Roche Diagnostics, Basel, Switzerland) as described previously.42
Statistical Analysis
The voxel-based morphometry analysis was performed by using the FSL software package (http://www.fmrib.ox.ac.uk/fsl/), according to the standard procedure. The essential processing steps included brain extraction with the FSL Brain Extraction Tool (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/BET), tissue-type segmentation with the FMRIB Automated Segmentation Tool (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/fast), nonlinear transformation into Montreal Neurological Institute reference space, and creation of a study-specific GM template to which the native GM images were then nonlinearly reregistered. The modulated segmented images were then smoothed with an isotropic Gaussian kernel with a σ of 2 mm. Finally, the voxelwise FSL General Linear Model (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/GLM) was applied by using permutation-based nonparametric testing with the FSL Randomize tool (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/Randomise/) with threshold-free cluster enhancement correction for multiple comparisons,43 considering fully corrected P values < .05 as significant. The analysis was performed to compare regions and APOE genotypes with the percentage of GM as a dependent variable with age, sex, and education as potential confounders. Furthermore, we created a mask for the bilateral mesial temporal cortex, posterior cingulate cortex, hippocampus, amygdala, caudate nuclei, and parietal and occipital lobes that was then applied to the GM image of the study-specific template.
Demographic and neuropsychological data were compared among groups by using regression models with group (stable versus deteriorating), APOE genotype (3/2, 3/3, 4/3), and a group × genotype interaction term as predictors. Logistic, ordered logistic, and linear regression models were used for binary, ordinal, and respectively continuous variables. Group effects (stable versus deteriorating) were compared by using the Z-test for plain and ordered logistic regressions and the t test for linear regression.
Logistic regression models were built to assess the relationship between cognitive status (dependent variable) and APOE genotyping, GM densities in the 3 areas of interest (posterior cingulate, hippocampus, mesial temporal lobe), age, sex, and education levels (independent variables). Because GM density values were all ranging between 0.32 and 0.87, we transformed them by using z scores (subtracting the overall mean and dividing by the overall SD) and thus calculated the standardized odds ratio, which represents the decrease in estimated risk for a 1-SD change in GM density.
Results
Demographic and Neuropsychological Data
The final sample included 382 subjects with 3D T1 scans available and APOE genotyping. We grouped these subjects into 3 groups: 43 with APOE*E2 (mean age, 74.1 ± 3.8 years; 22 with sCON and 21 with dCON), 274 with APOE*E3 (mean age, 74.1 ± 4.1 years; 132 with sCON and 142 with dCON), and 65 with APOE*E4 (mean age, 73.6 ± 4.1 years; 27 with sCON and 38 with dCON) genotypes. Demographic data and neuropsychological performances at baseline did not differ between sCON and dCON groups (data not shown). As expected, there were group differences at follow-up, with worse cognitive performances of dCON for the Trail-Making Test B (P = .015), Verbal Fluency (P = .045), and the Wisconsin Card Sorting Test (number of categories) (P = .034). The APOE*E4 allele had a negative impact on verbal fluency performance at follow-up (P = .011) with a significant group × APOE interaction (P = .003 for dCON). There was no other significant association between APOE genotyping and neuropsychological performances at follow-up (Table 1 and On-line Table).
Essential demographic data of the included study groups of stable and deteriorating elderly control participantsa
Whole-Brain Voxel-Based Morphometry
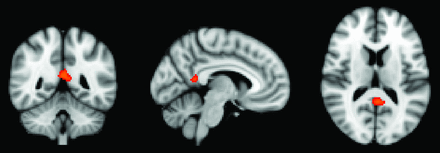
In a first step, we performed a voxelwise analysis across the entire brain. The posterior cingulate cortex demonstrated a significant difference between APOE*E3 > APOE*E4 for the dCON group (Fig 1).
Whole-brain voxel-based morphometry analysis demonstrating higher GM density for the comparison of APOE*E3 > APOE*E4 in the posterior cingulate cortex. P < .05 corrected.
ROI Analysis: APOE Effect on GM Densities in dCON and sCON
In a second step, we additionally performed an ROI analysis in 7 regions of particular interest in the context of cognitive decline (posterior cingulate cortex, hippocampus, mesial temporal lobe, parietal lobe, amygdala, and caudate nucleus) with the occipital lobe as a control region.
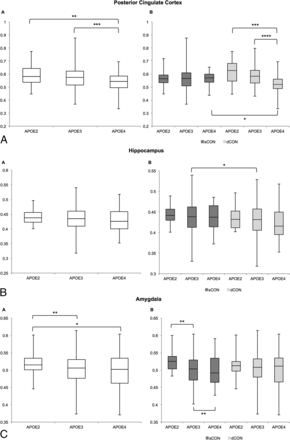
In the posterior cingulate, the GM concentration decreased from E2 to E3 to E4 across all participants. When we separated the patients into stable-versus-deteriorating controls, this decrease in GM concentration was present in only the dCON group (Fig 2A and Table 2). Moreover, we found a significant difference between sCON versus dCON in APOE*E4-positive individuals. Concerning the hippocampus, patients with dCON had lower GM concentrations compared with those with sCON, which was significant only in the APOE*E3 group, presumably due to the large sample size (Fig 2B and Table 3). In the amygdala, there was a decrease in GM concentration from APOE*E2 to APOE*E3 to APOE*E4 in all participants, and this effect was present only in the sCON but not in the dCON subgroups (Fig 2C and Table 3). In the parietal lobe, the pattern was inverse, with increased GM concentration in those with dCON, notably in those with APOE*E3 and APOE*E4 (Fig 2D and Table 3). No significant differences were found in the mesial temporal lobe, occipital lobe, or caudate nuclei (Fig 2E–G and Table 3).
ROI analysis of GM density depending on APOE status for the 7 target regions for all elderly controls (left-hand side) and separately for subjects with sCON and dCON (right-hand side). One asterisk indicates P < .05; 2 asterisks, P < .01; 3 asterisks, P < .001; 4 asterisks, P < .0001.
ROI analysis of GM concentration in the 7 regions for the comparison of sCON versus dCONa
ROI analysis of GM concentration in the 7 regions for the comparison of APOE*E2 versus APOE*E3 and APOE*E2 versus APOE*E4 and APOE*E3 versus APOE*E4
Logistic Regression Models
Higher GM densities in the hippocampus, middle temporal lobe, and amygdala were all associated with a decreased risk for dCON status at follow-up (hippocampus: standardized OR = 0.75; 95% CI, 0.61–0.92; P = .006; middle temporal lobe: standardized OR = 0.80; 95% CI, 0.65–0.98; P = .028; and amygdale: standardized OR = 0.75; 95% CI, 0.61–0.93, P = .008). Although significant, these associations explained <1.5% of cognitive variability. APOE genotyping, age, sex, and duration did not predict dCON status at follow-up. When categorizing GM densities into quintiles, we confirmed the assumption of a linearity of their association with the log odds, because the odds ratios displayed a gradient that is statistically significant for quintile 4 (threshold ≥ 0.4426118) and quintile 5 (threshold ≥ 0.48496512), with ORs of 0.51 (P = .044) and 0.44 (P = .013).
Discussion
Several studies demonstrated that the presence of the APOE*E4 allele modulates the expression of brain atrophy in MCI and clinically overt AD, increasing the vulnerability of the areas prone to early neurodegeneration such as the hippocampus, amygdala, and mesial temporal lobe.15⇓–17 Although higher cortical amyloid β load and decreased metabolism in the above-mentioned areas were reported in the APOE*E4 allele, cross-sectional MR imaging studies failed to identify consistent GM decreases associated with this genotype in elderly controls (for a review see Fouquet et al44). The finding in the current investigation that APOE*E4 was related to GM loss in only the subsequently deteriorating but not in the cognitively stable groups might explain these partially conflicting results of previous studies, which typically do not include detailed neuropsychological assessment and follow-up.
The present data show that the APOE*E4 genotype is not associated with an increased risk of dCON status at follow-up. Consistent with previous reports in elderly controls,15,18,20 no APOE genotyping–related effect was identified in the hippocampus and mesial temporal cortex in subjects with both sCON and dCON. In agreement with previous observations in this field, these observations indicate that the APOE*E4 allele detrimental effect in terms of structural changes and clinical progression becomes evident only in elderly individuals with significant cognitive deterioration (MCI) or clinically overt symptoms of dementia (early AD).14⇓⇓–17
The posterior cingulate cortex is known to be affected early in the AD process with significant hypometabolism in cognitively healthy individuals and those with MCI (both converters and nonconverters; for review see Teipel and Grothe45). In more advanced stages of the degenerative process, this area exhibits subtle atrophy and hypometabolism in subjects with amnestic amyloid-negative AD.46 Rare cross-sectional studies addressed the impact of APOE genotyping on the structural and functional integrity of the posterior cingulate cortex. Early contributions from the Cardiovascular Health Study indicated an APOE*E4-independent age-related atrophy in the hippocampus and posterior cingulate cortex in healthy elderly controls.47 More recently, an altered energy metabolism was reported in this area in young adult APOE*E4 carriers.48 In the same line, Lu et al49 reported cortical atrophy in the right cingulate gyrus in cognitively intact APOE*E4 carriers. Our observations parallel these findings, suggesting that the presence of this allele may have a detrimental effect on GM density in this vulnerable area.
The strengths of the present study include its longitudinal design, large number of community-dwelling subjects, and detailed neuropsychological testing at inclusion and follow-up. However, some limitations should also be considered. First, in line with recent core clinical criteria for MCI,50 the identification of deteriorating controls was based on the objective decline in cognitive functions measured by using serial, comprehensive neuropsychological assessments. However, in the absence of longer follow-up and AD biomarker characterization at baseline (including PET amyloid scans or the CSF τ/amyloid β42 ratio), the cognitive fate of these individuals remains uncertain so that they cannot be a priori considered as subjects with incipient AD. Second, the rarity of APOE*E4 homozygotes precluded a detailed analysis of gene-dose effect on GM densities. Third, handedness was not considered as a covariate in our MR imaging analysis, which included both left- and right-handed controls.
Conclusions
Our data reveal that the presence of the APOE*E4 allele is associated with decreased GM density in the posterior cingulate cortex in dCON, a community-based group of elderly controls who subsequently had subtle cognitive decline at 18-month follow-up. This APOE effect was not identified in cognitively stable controls. Most important, the APOE*E4 allele has no impact on GM density in areas affected early by neurofibrillary tangle formation such as the hippocampus and mesial temporal lobe. These observations suggest that decreased GM density in the posterior cingulate cortex should be systematically detected among APOE*E4 controls because it could represent a structural marker preceding subtle cognitive deficits in the very early stages of the degenerative process.
Footnotes
Disclosures: Sven Haller—RELATED: Grant: Swiss National Foundation, Comments: grant SNF 3200B0–1161193 and SPUM 33CM30–124111* and an unrestricted grant from the Assocation Suisse pour la Recherche Alzheimer*. *Money paid to the institution.
This work is supported by Swiss National Foundation grants SNF 3200B0-1161193 and SPUM 33CM30-124111 and an unrestricted grant from the Assocation Suisse pour la Recherche Alzheimer.
Indicates open access to non-subscribers at www.ajnr.org
References
- Received September 25, 2016.
- Accepted after revision February 17, 2017.
- © 2017 by American Journal of Neuroradiology