Abstract
BACKGROUND AND PURPOSE: For patients with cerebral vasospasm refractory to medical and hemodynamic therapies, endovascular therapies often remain the last resort. Data from studies in large cohorts on the efficacy and safety of multiple immediate endovascular interventions are sparse. Our aim was to assess the feasibility and safety of multiple repeat instant endovascular interventions in patients with cerebral vasospasm refractory to medical, hemodynamic, and initial endovascular interventions.
MATERIALS AND METHODS: This was a single-center retrospective study of prospectively collected data on patients with cerebral vasospasm refractory to therapies requiring ≥3 endovascular interventions during the course of treatment following aneurysmal subarachnoid hemorrhage. The primary end point was functional outcome at last follow-up (mRS ≤2). The secondary end point was angiographic response to endovascular therapies and the appearance of cerebral infarctions.
RESULTS: During a 4-year period, 365 patients with aneurysmal subarachnoid hemorrhage were treated at our institution. Thirty-one (8.5%) met the inclusion criteria. In 52 (14%) patients, ≤2 endovascular interventions were performed as rescue therapy for refractory cerebral vasospasm. At last follow-up, a good outcome was noted in 18 (58%) patients with ≥3 interventions compared with 31 (61%) of those with ≤2 interventions (P = .82). The initial Hunt and Hess score of ≤2 was a significant independent predictor of good outcome (OR, 4.7; 95% CI, 1.2–18.5; P = .03), whereas infarcts in eloquent brain areas were significantly associated with a poor outcome (mRS 3–6; OR, 13.5; 95% CI, 2.3–81.2; P = .004).
CONCLUSIONS: Repeat instant endovascular intervention is an aggressive but feasible last resort treatment strategy with a favorable outcome in two-thirds of patients with refractory cerebral vasospasm and in whom endovascular treatment has already been initiated.
ABBREVIATIONS:
- CVS
- cerebrovascular vasospasm
- GCS
- Glasgow Coma Scale
- HH
- Hunt and Hess
- IAN
- intra-arterial nimodipine
- PTA
- percutaneous transluminal balloon angioplasty
- RCVS
- refractory CVS
Cerebral vasospasm (CVS) after aneurysmal subarachnoid hemorrhage remains a major cause of delayed cerebral ischemia and related morbidity and mortality.1,2 Standard treatment consists of hyperdynamic/hypertensive therapy and orally administered nimodipine to prevent ischemic events.3,4 Nevertheless, 20%–30% of patients develop refractory CVS with cerebral infarction causing permanent neurologic deficits or death.5 In cases of severe CVS and threatened ischemia despite hemodynamic and medical treatment, no commonly accepted treatment guidelines exist.6 Although endovascular methods offer some last resort treatment options, their use is generally not extended throughout the entire CVS course and application is limited to 1 or 2 or, at most, 3 interventions, resulting in an ambiguous functional outcome with most patients unable to resume work post-SAH.7⇓⇓–10
In this single-center audit of practice, we evaluated repeat immediate endovascular therapies in a subgroup of symptomatic patients with the most severe, refractory CVS during the vasospasm period.
Materials and Methods
Patient Inclusion Criteria and Study End Points
To comply with the inclusion criteria, patients with proved aneurysmal subarachnoid hemorrhage had to fulfill the requirement of medically and hemodynamic refractory CVS (rCVS) necessitating ≥3 endovascular interventions during the CVS period. These patients were compared with a cohort of patients with aneurysmal subarachnoid hemorrhage necessitating 1–2 endovascular interventions during the refractory CVS period. Patients with mycotic aneurysms were excluded from the study. The primary end point was functional outcome at last follow-up (modified Rankin Scale ≤2). The secondary end point was angiographic response to endovascular therapies and the appearance of cerebral infarctions.
Management of CVS
After early aneurysm treatment (≤24 hours with either clip or coil embolization), patients were continuously monitored in the intensive care unit and kept normovolemic with blood pressure at normal high levels. A daily check was made of electrolytes (ie, sodium), and an assessment with transcranial Doppler was performed. Medical therapy consisted of nimodipine administered by the enteral route (60 mg every 4 hours for 21 days). In conscious patients, immediate endovascular therapy was indicated in cases of symptomatic rCVS, defined as the appearance of a new neurologic deficit despite medical and hemodynamic treatment (ie, hyperdynamic/hypertensive therapy) or a decline in consciousness not resulting from hydrocephalus (ie, an NIHSS score increase of ≥2 and/or a Glasgow Coma Scale [GCS] score decrease of ≥2). In unconscious patients, rising transcranial Doppler mean velocities of ≥150 cm/s or increased flow velocity of ≥50 cm/s during 24 hours was considered an indication for endovascular treatment in cases in which the CT perfusion scan or MR imaging showed a mean transit time of >5.9 seconds.11 Other indications for endovascular rescue therapies included patients with marked changes in brain-tissue oxygen pressure values (<15 mm Hg, Licox Brain Oxygen Monitoring System [Integra LifeSciences, Saint Priest, France]) who were not responding to medical and hemodynamic therapies when the MR imaging showed a diffusion-perfusion mismatch.12,13
Endovascular Interventions
Intra-arterial nimodipine (IAN) with continuous infusion was delivered locally through a 0.18-inch microcatheter (Renegade Hi-Flo; Boston Scientific, Natick, Massachusetts) into the most affected intracranial vessel segments. A dose of 2.5 mg of nimodipine was administered at the most proximal site of each spastic artery (12 mL in 38-mL saline, administered at 20–30 mL per hour for 30 minutes). Repeat angiography was performed 30 minutes after IAN to evaluate the response of the spastic vessels. In cases of poor response to IAN, percutaneous transluminal balloon angioplasty (PTA) was performed by using intracranial balloon catheters (Gateway; Stryker, Kalamazoo, Michigan). Balloon diameter and inflation pressure were chosen according to the target vessel diameter before vasospasm. “Angiographic CVS” was defined as narrowing of the arterial diameter by ≥30% from the baseline diameter, as assessed by 2 experienced interventional neuroradiologists (G.S., J.G.).
Neurologic Outcome
The immediate response to IAN and/or PTA was evaluated by neurosurgeons and critical care physicians. In those patients who did not respond, MR imaging was performed. In cases of confirmed multiple ischemic areas without any salvageable penumbra, therapy was suspended.14,15 Complications resulting from SAH, obliteration procedures, and multiple interventions were recorded separately according to the clinical protocol. Neurologic outcome was assessed by mRS, the GCS, and Glasgow Outcome Scale at discharge and at the last follow-up.5 For patients who lacked long-term follow-up data, the mRS was assessed via telephone interview.16 The patients' morbidity and reintegration into the workplace at last follow-up were documented.
Assessment of Stroke
Radiologic ischemia was documented with CT or MR imaging.17 Twenty-four hours following radiologic or surgical aneurysm obliteration, a cerebral CT scan was obtained in all patients to look for treatment-related ischemia. CT scans or MR imaging at the time of patient discharge allowed the visualization of new stroke areas resulting from CVS during the clinical course. Associated functional deficits were registered.
Statistical Methods
Data were analyzed by using SPSS statistical software, Version 21.0 (IBM, Armonk, New York). Clinical outcome was dichotomized into favorable (mRS ≤2) and poor outcome (mRS ≥3). Data are given as mean ± SD unless otherwise stated. For comparisons of means between the 2 analysis groups (ie, <2 interventions versus ≥3 interventions), the Student t test was used for normally distributed data (comparing patient age and follow-up time), and the Mann-Whitney U test, for skewed or non-normal data (comparing the number of treatments, vessel segments treated, and vascular territories). The other categoric variables were compared between the 2 analysis groups by using the Pearson χ2 test or the Fisher exact test, as appropriate (Tables 1 and 2). To identify predictors of good functional outcome (mRS ≤2), we performed multivariable analysis with a binary logistic regression model, including the following variables: patient age, sex, Hunt and Hess (HH) grade after SAH, the number of treatments, the number of vessels treated, the number of vascular territories, aneurysm location, further aneurysms, aneurysm obliteration, necessity for PTA, cerebral infarcts, and length of follow-up. Odds ratios and 95% CI were calculated. P values ≤ .05 were considered statistically significant.
Characteristics of patients with cerebral vasospasm treated with ≥3 interventions compared with those treated with ≤2 interventions
Primary and secondary outcome measurements following multiple interventions
Results
Patient Demographics
During the 4-year study period, 365 patients with aneurysmal SAH were admitted to our institution. Thirty-one (8.5%) met the inclusion criteria with ≥3 endovascular interventions during the CVS period. In 52 patients with aneurysmal SAH, 1–2 endovascular interventions were performed during the CVS period. Patient characteristics did not differ significantly between the 2 cohorts and are summarized in Table 1 (see also On-line Table 1). Mean age was 54 years (range, 24–77 years), and 66 (80%) were women. Forty-five (54%) patients presented with a Hunt and Hess grade of ≥3.18 At admission, a mild GCS score of 14–15 was noted in 42% of patients; a moderate score (GCS, 9–13), in 21%; and a severe score (GCS, 3–8), in 38%. Most patients (92%) presented with a Fisher grade 319 at clinical onset. Patients with aneurysms not suitable for coil embolization were treated with clip reconstruction (29% of patients) based on interdisciplinary review. Thirty-six percent of patients presented with multiple aneurysms. In 65% of patients, the ruptured aneurysm was located in the anterior, and in 35%, in the posterior circulation. In 51 patients (63%), an external ventricular drain was inserted due to acute hydrocephalus.
Endovascular Interventions
The number of treatments, vessel segments treated, and vascular territories and the requirement for PTA were significantly higher in patients with ≥3 interventions than in patients with ≤2 interventions (Table 1). Endovascular treatment for CVS was performed between 3 and 15 days after the initial ictus (mean, 6 days). The mean clinical course of vasospasm lasted for 4.8 ± 2.8 days (range, 1–14 days); in 15 patients (48%), it lasted for >5 days. A total of 210 (mean, 2.5 ± 1.7 per patient) endovascular interventions were performed (mean, 4.3 ± 1.6 in patients with ≥3 interventions, versus mean, 1.5 ± 0.5 in patients with ≤2 interventions; P < .001), resulting in the treatment of 450 vessel segments (mean, 5.4 ± 5.3 per patient; range, 1–40 segments; mean, 9.6 ± 6.6 in patients with ≥3 interventions, versus mean, 2.9 ± 1.5 in patients with ≤2 interventions; P < .001), and 157 vascular territories (mean, 1.9 ± 0.8 per patient; range, 1–3; mean, 2.3 ± 0.8 in patients with ≥3 interventions, versus mean, 1.6 ± 0.7 in patients with ≤2 interventions; P < .001). A moderate immediate angiographic effect (arterial dilation <30%) was observed after IAN in 26 (31%) interventions, and consecutive transluminal PTA was applied in these cases, namely in 9 (17%) patients with ≤2 interventions versus 17 (55%) patients with ≥3 interventions (P = .001). In the cohort of patients with ≥3 interventions, 13 (42%) underwent 3 treatment sessions, 8 (26%) underwent 4 sessions, 4 (13%) underwent 5 sessions, and 4 (13%) underwent 6 sessions (Fig 1). One patient had a total of 7 treatment sessions, and another, 10 sessions during the CVS course. Of the 52 patients with ≤2 interventions, 26 (50%) underwent 1 treatment session and 26 (50%), 2 sessions.
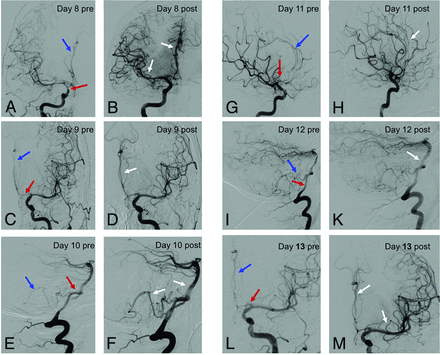
Repeat angiograms in refractory cerebral vasospasms. Repeat angiograms (red arrows: proximal spastic vessels; blue arrows: distal spastic vessels) in a 41-year-old woman with a ruptured anterior communicating artery aneurysm initially treated with coil embolization. On day 8 after the ictus, the patient presented with hemiparesis on the left side. A, ICA angiogram, anteroposterior view, shows vasospasm of the right-sided M1 and A1/A2 segments. B, ICA angiogram of the same patient after nimodipine infusion demonstrates reduced vasospasm of the M1 and A1/A2 segments, with improved perfusion of both the proximal and distal arteries. C–M, Corresponding angiograms of the anterior circulation (C, D, G, H, L, M) and posterior circulation (E, F, I, K) in the same patient during the CVS period from day 9 to day 13. Images on the right side show the effect after IAN. Note the effect of nimodipine infusion on the vessel diameter (white arrows).
Functional Outcome
Functional outcome was recorded at discharge (mean, 3 weeks; range, 2–6 weeks) and after a median follow-up at 10 months (range, 1–24 months; Table 2 and On-line Table 2). A good functional outcome (median mRS ≤2) at last follow-up was recorded in 49 patients (60%); a poor outcome (median mRS 3–6), in 33 patients (40%), with no statistically significant difference between the 2 cohorts (P = .82). Persistent disabilities requiring constant nursing care (mRS 4–5) were noted in 4 patients (8%) with ≤2 interventions and in 3 (10%) of those with ≥3 interventions. Overall mortality (mRS 6) was 20%, with no differences between the 2 cohorts (P = .31).
Sixty-one percent of patients rated headache/fatigue and 39%, a persisting neurologic deficit as the most disturbing long-term effect. In patients with ≥3 interventions, none of the working-age patients were able to work full-time at long-term follow-up, 42% worked part-time either in the same or another profession, 23% had no employment, and early retirement was taken by 3%. In contrast, in patients with ≤2 interventions, 5 (6%) of the working-age patients were able to work full-time, whereas 6 (13%) worked part-time either in the same or another profession. Sixteen (33%) patients had no employment at last follow-up.
The following variable was associated with a good functional outcome (median mRS 0–2) at last follow-up (Table 3): Hunt and Hess scale of ≤2 (OR, 7.6; 95% CI, 2.7–22.3; P <.001), whereas new cerebral infarctions (OR, 14.8; 95% CI, 3.9–55.3; P < .001) and infarction in eloquent areas (OR, 26.0; 95% CI, 7.7–88.2; P < .001) were associated with a poor outcome (median mRS >2). Multivariable analysis revealed the HH scale score of ≤2 (OR, 4.7; 95% CI, 1.2–18.5; P = .03) as an independent predictor of favorable functional outcome (mRS ≤2) and infarcts in eloquent areas (OR, 13.5; 95% CI, 2.3–81.2; P = .004) as an independent risk factor for poor outcome (mRS 3–6) at last follow-up.
Factors associated with good functional outcome (mRS ≤2) at last follow-up
Cerebral Infarctions
Assessments of new infarcted areas and infarcts in eloquent regions are shown in Table 2 and On-line Table 2. New infarcted areas at discharge were registered in 23 (74%) patients with ≥3 interventions compared with 26 (52%) patients with ≤2 interventions (P = .06). There was no difference between the 2 cohorts regarding infarcts located in eloquent brain areas, which were observed in 17 (55%) patients with ≥3 interventions versus 18 (37%) patients with ≤2 interventions (P = .17). We identified the following risk factors associated with infarction at discharge (Table 4): number of treatments (OR, 1.4; 95% CI, 1.0–1.9; P = .05), number of vessels treated (OR, 1.2; 95% CI, 1.0–1.4; P = .03), and requirement for PTA (OR, 2.7; 95% CI, 1.0–7.9; P = .06). Multivariable analysis revealed the HH scale score of ≥3 (OR, 3.4; 95% CI, 1.2–9.3; P = .02) and the number of vascular territories treated (OR, 3.1; 95% CI, 1.3–7.3; P = .01) as independent predictors of infarcts at discharge.
Risk factors associated with infarction at discharge
Treatment-Related Morbidity and Mortality
There was no treatment-related mortality. Complications resulting from aneurysm obliteration procedures consisted of 2 (2%) patients with thrombosis of the parent vessel during coil embolization, which dissolved following intra-arterial thrombolytic therapy. In 1 patient (1%), aneurysm rerupture during coil embolization occurred but did not result in a clinical decline.
Complications resulting from spasmolysis therapies consisted of dissection in the access vessel of the ICA in 1 (2%) patient with ≤2 interventions versus 5 (16%) patients with ≥3 interventions (P = .03), requiring stent placement for persistent high-grade stenosis in 1 patient. Another patient (1%) developed a pseudoaneurysm at the puncture side of the femoral artery.
Discussion
IAN offers some last resort treatment strategies, yet application generally remains limited to 1 or 2 or, at most, 3 interventions.20⇓⇓–23 Cases of patients undergoing >3 endovascular interventions in response to persistent, recurrent, or worsening CVS have seldom been described, and results on functional outcome remain ambiguous.24 Jun et al25 reported a subgroup of patients (6%, 12 of 189) with rCVS who underwent ≥3 endovascular interventions, and Pandey et al, 26 a subgroup of 7% of patients (2 of 27); but no functional outcome was reported in this subgroup of patients. Most interesting, in the study by Pandey et al, nicardipine was infused through a cervical catheter, with selective catheterization and angioplasty reserved for refractory cases, thereby reducing the risk related to microcatheterization itself.26 In their series, no complications attributed to the angiographic interventions were described. Given that the rate of cervical artery dissections was higher than that recorded in other studies,25,27 less aggressive approaches might be considered in selected cases.
Due to the transient vasodilatory effects of IAN,23,28⇓⇓–31 Albanese et al7 reported prevention of new ischemic events in 9 of 12 patients with prolonged intra-arterial infusion of verapamil. However, catheterization over an extended period might increase the risk of thrombus formation and subsequent embolisms.29 Additionally, it generally involves different personnel in the intensive care unit, thereby increasing the risk of air embolism by manipulating the perfusion system. Indeed, repeat endovascular procedures via microcatheterization had greater risks for related complications but also allowed immediate initiation of endovascular therapies, which has been shown to markedly improve outcome compared with patients whose treatment was delayed.32
Depending on the location and severity of the CVS, either PTA or IAN can be used in the clinical setting. Balloon angioplasty is indeed effective in treating proximal artery vasospasms and results in more durable clinical improvement.25,33,34 However, in smaller and more distal vessels, this technique is limited by the possibility of vessel rupture.27 For more distal or diffuse vasospasms with reduced parenchymal perfusion, several superselective intra-arterial agents have been shown to be effective though transient.35 A number of clinical studies regarding the effect of intra-arterial nimodipine in patients with severe refractory CVS exist8,9,36; however, some controversy remains as to the effectiveness of IAN.37 Furthermore, these reports comprise mostly small, retrospective case series.38
While we noted a good clinical outcome in 61% of patients with ≤2 interventions versus 58% with ≥3 interventions (P = .82) after a median follow-up of 10 ± 8 months, 70% of patients recruited for the Barrow Ruptured Aneurysm Trial (BRAT) showed good functional outcome after 1 year,39 67% after 3 years,40 and 62% after 6 years following SAH.41
In patients recruited for the International Subarachnoid Aneurysm Trial (ISAT), an mRS of ≤2 after 1 year was reported in 72.8% of them and in 70.2% 8 years later.42⇓–44 However, the BRAT and ISAT trials represent the full range of patients, including those with a good-grade SAH,45 thus not reflecting the subpopulation at highest risk for refractory CVS with subsequent infarction and poor quality of life.46
Most important, a poor neurologic outcome (mRS 4 or 5) with persistent disabilities requiring constant nursing care could be prevented in most patients. Pegoli et al46 noted an excellent outcome (mRS 0–1) in 63% of patients with aneurysmal SAH; however, symptomatic CVS and consecutive infarctions were risk factors for poor outcome.46,47
As many as 91% of patients with poor quality of life following SAH failed to return to full-time work.48 Aneurysmal SAH is a cerebrovascular disease particularly affecting the younger working-age population, thereby increasing the economic burden for numerous patients, their families, and society as a whole.49,50 At last follow-up, only 6% of patients worked full-time, 24% of patients worked part-time, and 29% were unemployed. Our results are in line with these earlier findings. Indeed, vasospasm-related delayed ischemic neurologic deficits and cerebral infarction are strongly associated with unfavorable outcomes following SAH.15,51,52 While cerebral or angiographic vasospasm is a treatable condition, the effectiveness of nimodipine, hemodynamic therapy, and endovascular interventions, including angioplasty, in preventing neurologic deficits remains controversial.53⇓–55
In summary, our approach of immediate repeat endovascular interventions in patients with the most severe CVS refractory to traditional therapies resulted in a favorable outcome in about two-thirds of patients and prevented severe disability in most of them. However, it remains difficult to assess whether immediate endovascular treatments ultimately result in a better neurologic outcome because vasospasm is not the only cause of delayed ischemic complications.56
Nevertheless, rescue therapies to reduce the impact of rCVS following SAH might be beneficial in devastating cases. On the basis of the current data and from the experience we gained from performing these procedures in this subpopulation of patients at highest risk for refractory CVS, we think that it is important to continue the effort to treat even those patients who are refractory to 1 or 2 endovascular interventions, even when little or no effect and ongoing cerebral vasospasm are evident. Large multicenter, prospective, randomized controlled studies are, however, needed to prove the effectiveness of repeat instant endovascular interventions in patients with refractory cerebral vasospasms—not an easy undertaking given the ethical problems of withholding therapeutic interventions, even if the effect of the therapy remains ambiguous. At present, treatment of rCVS with immediate multiple endovascular interventions is feasible and safe and may be warranted until the significance of these interventions becomes better understood.
Study Limitations
Assessment of cerebral infarctions by CT in 36% of all patients may actually lower the true rate of radiographically detected cerebral infarctions, given the higher sensitivity of MR imaging in detecting vasospasm-related infarction.17 In addition, a median follow-up of 10 ± 7.0 months may under-represent the true rate of poor outcome, considering longer term outcome studies that showed a steady increase in patients with poor outcome across time.39,41
Conclusions
The use of immediate repeat endovascular interventions is an aggressive but feasible last resort treatment strategy effective for a selected subgroup of patients who fail to respond to traditional CVS therapies and endovascular interventions.
Acknowledgments
The assistance of Ms Susan Kaplan in preparing this manuscript is acknowledged. We wish to thank Dr Corrado Bernasconi for statistical advice.
Footnotes
Disclosures: Lukas Andereggen—UNRELATED: Grants/Grants Pending: Swiss National Science Foundation. Werner Z'Graggen—UNRELATED: Employment: Bern University Hospital. Gerhard Schroth—UNRELATED: Employment: Bern University Hospital, Comments: normal salary as a 50% senior consultant of the University Institute for Diagnostic and Interventional Neuroradiology of Bern University Hospital Inselspital; Grants/Grants Pending: Swiss National Science Foundation, Comments: grant for neuropsychological investigations before and after carotid stenting*; Payment for Lectures including Service on Speakers Bureaus: European Stroke Organisation, Master Course at the Danube University in Krems, Austria, Comments: lectures and hands-on workshops; money paid exclusively to the Inselspital. Michael Reinert—UNRELATED: Consultancy: Brainlab, DePuys Synthes*; Employment: Ente Ospedaliero Cantonale Ticino; Grants/Grants Pending: Lega Ticinesi contro il Cancro*; Payment for Lectures including Service on Speakers Bureaus: Brainlab.* Jan Gralla—UNRELATED: Consultancy: Covidien, Comments: former Global Principal Investigator for the Star Study on thrombectomy in acute stroke, consultant for Covidien.* *Money paid to the institution.
The authors declare no conflict of interest. This study was approved by the Ethics Committee of the Canton of Bern, Switzerland (Kantonale Ethikkommission Bern, KEK, 177/14).
Paper previously presented in part at: Vasospasm 2013: The 13th International Conference on Neurovascular Events after Subarachnoid Hemorrhage, Lucerne, Switzerland; July 10–12, 2013.
References
- Received April 21, 2016.
- Accepted after revision October 11, 2016.
- © 2017 by American Journal of Neuroradiology