Abstract
BACKGROUND AND PURPOSE: Intra-arterial chemotherapy for retinoblastoma is not always a straightforward procedure, and it may require an adaptable approach. This study illustrates strategies used when the ophthalmic artery is difficult to catheterize or not visible, and it ascertains the effectiveness and safety of these strategies.
MATERIALS AND METHODS: A retrospective study was performed on a series of 108 eyes affected by intraocular retinoblastoma and selected for intra-arterial chemotherapy (follow-up range, 6–82 months). We recognized 3 different patterns of drug delivery: a fixed pattern through the ophthalmic artery, a fixed pattern through branches of the external carotid artery, and a variable pattern through either the ophthalmic or the external carotid artery.
RESULTS: We performed 448 sessions of intra-arterial chemotherapy, 83.70% of them through the ophthalmic artery and 16.29% via the external carotid artery. In 24.52% of eyes, the procedure was performed at least once through branches of the external carotid artery. In 73 eyes, the pattern of drug delivery was fixed through the ophthalmic artery; for 9 eyes, it was fixed through branches of the external carotid artery; and for 17 eyes, the pattern was variable. Statistical analysis did not show any significant difference in the clinical outcome of the eyes (remission versus enucleation) treated with different patterns of drug delivery. Adverse events could not be correlated with any particular pattern.
CONCLUSIONS: Alternative routes of intra-arterial chemotherapy for intraocular retinoblastoma appear in the short term as effective and safe as the traditional drug infusion through the ophthalmic artery.
ABBREVIATIONS:
- ECA
- external carotid artery
- FPEC
- fixed pattern through the ECA
- FPO
- fixed pattern through the OA
- IAC
- intra-arterial chemotherapy
- MMA
- middle meningeal artery
- OA
- ophthalmic artery
- VP
- variable pattern
Intra-arterial chemotherapy (IAC) is a therapeutic strategy devised for the local treatment of primary and metastatic tumors.1 At first, its application was mainly for treatment of hepatocellular carcinoma or hepatic metastasis of tumors of the digestive tract. However, this approach rapidly spread from the liver to several extrahepatic sites.1 In the orbit, one of the most striking applications, in terms of success rate, was certainly the infusion of antitumoral drugs through the ophthalmic artery (OA) to treat intraocular retinoblastoma.2⇓⇓⇓⇓⇓–8 Across time, the technique of delivering drugs intra-arterially to the eye has been refined. Balloon occlusion of the internal carotid artery just distal to the OA origin4 has been rapidly replaced by the direct catheterization of the OA.3,5,8
However, in some cases, the procedure is not as straightforward as expected because of difficulties in catheterizing the OA or because angiographic visualization of the OA fails altogether.5,7⇓–9 Two alternative techniques have been previously proposed in such cases: The first one involves the superselective catheterization of the middle meningeal artery (MMA), provided that an anastomosis between this vessel and the OA allows an acceptable choroidal blush; the second one is to just reapply the balloon technique to the ICA.5,9 Failure to catheterize or infuse the OA can be a temporary occurrence, and the procedure can be successfully performed sometime later8; on the other hand, a successful procedure through the OA on 1 or >1 occasion does not guarantee the same achievement in the following scheduled sessions of IAC.9
We observed that in a remarkable percentage of children, the technical success of the treatment required different routes of drug infusion, depending on anatomic variants or on temporary hemodynamic variations. In some cases, the route of administration of the drugs had to be changed even within the same procedure so that the antitumoral agents were delivered in part through 1 artery and in part via another one. In our experience, in children, hemodynamics are not as stable as in adults, so the blood flow in the OA is the result of a balance between the external and the internal carotid systems, which is susceptible to temporary changes. Accordingly, an adaptable approach may be required to successfully deliver antitumoral drugs to the affected eye. Here, we report our experience based on a retrospective review of 108 eyes affected by retinoblastoma and selected for intra-arterial infusion of melphalan or melphalan plus topotecan. We report the alternative strategies and routes of administration used along with an evaluation of the clinical outcome of eyes treated via the external carotid artery (ECA) versus eyes treated through the ICA.
Materials and Methods
From June 2008 to November 2014, a series of 99 patients with retinoblastoma (44 females, 55 males; age range at first treatment, 5–108 months) in 108 eyes were scheduled for superselective ophthalmic artery infusion of melphalan alone or in association with topotecan. The minimum follow-up was 6 months (up to 82 months). A multidisciplinary group jointly discussed the opportunity for treatment and obtained informed consent. Inclusion criteria were newly diagnosed unilateral or bilateral groups C–D retinoblastomas; unilateral groups A–B with macular involvement; and unilateral or bilateral groups A–D retinoblastomas with partial remission and/or relapse after systemic chemotherapy and/or focal therapies including cryotherapy, laser ablation, brachytherapy and external beam radiation therapy, intravitreal melphalan, or a combination of these. Exclusion criteria were diffuse infiltrating retinoblastoma, anterior chamber invasion, secondary glaucoma, vitreous hemorrhage, optic nerve infiltration, diffuse choroidal infiltration, scleral infiltration, extraocular disease extent, and intracranial metastatic disease at gadolinium-enhanced MR imaging of the head and orbits. Ophthalmic evaluations were performed 1–7 days before and 3 weeks after each treatment and included external examination; visual acuity testing when possible, depending on the patient's age and cooperation; pupil and eye motility evaluation; and complete fundus examination. All ophthalmic evaluations were performed with the patient under anesthesia, and included RetCam digital photography (Clarity Medical Systems, Pleasanton, California) and B-scan sonography at 12 MHz. Grading was based on the tumor volume at RetCam digital photography at the beginning of chemosurgery and after each treatment follow-up examination as previously reported.10
After the final session, we evaluated the effectiveness of the treatment, measuring the ophthalmologic response (ie, the rate of reduction of the tumor volume). A decrease of <50% of the volume (0%–49%) was considered a low response; a medium response was achieved when the decrease was between 50% and 79% of the volume; and a decrease between 80% and 100% of the volume was considered a high response. The follow-up schedule consisted of clinical, ophthalmic, and eventually MR imaging evaluation, every 4–6 weeks for 6 months, every 6–8 weeks for the following 6 months, and every 8–10 weeks starting from the second year.
Angiographic Procedure
All patients underwent general anesthesia with intubation. A 4F micropuncture set was used to access the common femoral artery with subsequent placement of an arterial sheath. The patient was then anticoagulated with intravenous heparin (70 IU/kg, followed by a continuous infusion of 10–15 IU/kg/h). A selective angiography of the ICA was always performed at the beginning of each session of treatment. The micronavigation of the ICA and direct catheterization of the OA ostium by using a flow-directed straight microcatheter (Magic 1.5 or Baltacci 1.2; Balt, Montmorency, France; or UltraFlow 1.5, Covidien, Irvine, California) were always attempted before considering alternative routes of IAC. When required, vasospasm reactions were solved with the infusion of nimodipine. If an adequate choroidal blush was unattainable via the direct catheterization of the OA (On-line Video), alternative routes of IAC delivery through the anastomoses with the ECA were sought. A detailed description of our procedure has been previously reported.8 When the OA was visible but its entrance was too angulated for regular catheterization, we customized the tip of the microcatheter, shaping it to properly fit the anatomy of the patient.8 In a few selected cases, embolization of some branches of the ECA with a mixture of a cyanoacrylate-based synthetic and Lipiodol (Guerbet, Roissy, France), administered through a flow-directed microcatheter (Magic 1.2, Balt), was performed. The balloon technique was never applied in our patients.
The dose of melphalan was chosen according to the patient's age and the size of the globe at sonography,5,8,11 ranging from 3 to 6 mg/mL and possibly associated with 0.3 or 0.4 mg of topotecan (based on the patient's age). Drugs were injected by a 30-minute pulsatile infusion to avoid streaming and nonhomogeneous drug delivery.8 After delivering a half dose and at the end of the procedure, we checked blood flow efficiency by the microcatheter under fluoroscopy to confirm hemodynamic stability.
A final lateral arteriogram of the ICA or common carotid artery (depending on the pathway of drug delivery) was performed to rule out any procedure-related complications such as vasospasm, embolism, or arterial dissection.
Once the session was completed, the catheters were removed, the femoral sheath was pulled, and hemostasis of the groin puncture was achieved by manual pressure. Anesthesia was discontinued, and the child was kept under observation for 24 hours before discharging.
Any angiographic procedural complications, as well as systemic and local adverse events, were noted on the patient's medical record after each treatment session and during follow-up.
Retrospective and Statistical Analysis
To compare the clinical outcome and the safety of the IAC conveyed via branches of the ECA, we retrospectively divided the treated eyes into 3 groups according to the technique of drug delivery during the entire treatment: Group 1 consisted of eyes that received IAC with a fixed pattern of drug delivery through the OA (FPO) (ie, they were always treated through the OA); group 2 included eyes with a fixed pattern through the ECA (FPEC) (ie, they were treated consistently through branches of the ECA); and group 3 referred to eyes that received IAC at times via the OA and at times via branches of the ECA, describing a variable pattern (VP) of drug delivery (interprocedural variability). A χ2 test was used to verify whether the proportion of eyes that received pretreatment of any sort before undergoing IAC or that were treated with just melphalan (monotherapy) were the same in the 3 groups. Data on the ophthalmologic response of the eyes treated in the 3 groups and data on the adverse events were compared by means of the χ2 test. Significant age differences in patients in the 3 groups and differences in radiation exposures were verified with ANOVA tests. A P < .05 was considered statistically significant for all tests.
Results
From June 2008 to November 2014, 99 patients were selected for IAC. Because 9 patients were affected bilaterally, the total number of treatable eyes was 108. Two patients among the first 10 treated did not receive IAC due to technical failure. Overall, 106 eyes were treated, and we performed 448 sessions of IAC, 375 of them (83.70%) through the OA and 73 (16.29%) via branches of the ECA. In 26 eyes (24.52% of cases), IAC was performed at least once through branches of the ECA by using 1 of the many anastomoses that connect this artery with the OA.12
When the IAC was performed through the ECA, the branch most frequently used to deliver the antitumoral drugs was the MMA. This artery was used, often more than once, in 24 orbits for 64 sessions of IAC. Overall, the MMA was catheterized in 87.67% of the sessions in which IAC was performed through the ECA. However, several anastomoses connecting the MMA with the OA were used for drug delivery: the direct anastomosis between the 2 vessels (meningo-ophthalmic artery) was used in 14 eyes (38 sessions); the anastomosis between the MMA and the lacrimal artery was used in 9 orbits (24 sessions); and finally, in 1 orbit (2 sessions), we used an unusual anastomosis between the MMA and the supraorbital artery.12
Less frequently, the arteries used for delivering the antitumoral drugs were periorbital vessels that anastomosed with arteries running in the anterior orbit and were identified following previously published criteria,13 such as the dorsal nasal artery anastomosed with the angular artery (1 eye, 1 session),12 the frontal branch of the superficial temporal artery that anastomosed with the supratrochlear artery (1 eye, 1 session),12 the zygomatico-orbital artery anastomosed with the lacrimal artery (1 eye, 2 sessions),12 and the anterior deep temporal artery connected to the lacrimal artery (3 eyes, 5 sessions).12
Seven patients (7 eyes affected by grade D tumors) abandoned the treatment after just 1 session (1 patient treated via a branch of the ECA and 6 via the OA). In 4 of these 7 patients, the ophthalmologic evaluation showed a poor response or the progression of the disease; in the remaining patients, a massive tumor necrotic fragmentation involving the whole vitreous cavity occurred. The remaining 90 patients (99 eyes) underwent from 3 to 6 sessions of IAC. For some eyes, the technique of drug delivery was consistent throughout all cycles of treatment (FPO or FPEC); for others, it could vary from session to session or even within the same session (VP) (Fig 1). As expected, the most frequent pattern of drug delivery was the FPO, observed for 73 eyes (73.74%). For 9 eyes (9.1%), the pattern of drug delivery was the FPEC. The VP was used in 17 eyes (17.2%). Eyes that were pretreated before undergoing IAC were 52%, 33%, and 41%, respectively, in groups 1, 2, and 3. On the other hand, the proportion of eyes treated in monotherapy with melphalan in groups 1, 2, and 3 were respectively 45%, 55.5%, and 76.5%. The χ2 test did not show any statistically significant association between pretreatments or monotherapy and the pattern of drug delivery (P > .05). The mean age distribution of the patients in the 3 groups was 33.84 ± 42.47 months in group 1, 21.55 ± 10.26 months in group 2, and 33.11 ± 10.26 months in group 3. Statistical analysis did not show any significant age difference among groups (P > .05).
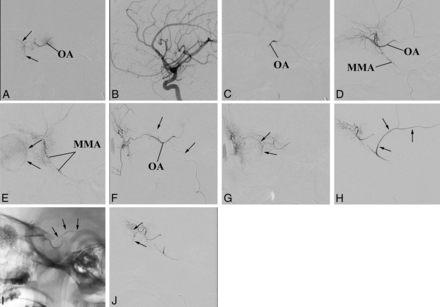
An exemplary case of adaptable approaches to the IAC. The patient has bilateral retinoblastoma previously enucleated on 1 side (the prosthesis is visible). The remaining eye is a case of VP of drug delivery. A, The OA is successfully catheterized and used in 2 sessions of IAC because the choroidal blush (arrows) is regularly achieved. B–E, Third session of IAC. Selective angiography of the ICA does not show any visible OA (B). Nevertheless catheterization of the OA is successful (C), though the contrast medium flows back into the ICA (On-line Video). Superselective angiography of the MMA shows a good anastomotic pathway to the OA (D), which allows achieving the choroidal blush (arrows in E). F and G, Fourth session of IAC. This time the anastomosis between the MMA and the OA does not guarantee the choroidal blush. An alternative route for drug delivery through the ECA is sought and found between the frontal branch of the superficial temporal artery (arrows point to the microcatheter within the artery) and the supratrochlear artery (F). A later angiographic phase shows that this pathway guarantees the choroidal blush (arrows) (G). H–J, Fifth session of IAC. The anastomosis between the MMA and the OA is working again. However, the contrast medium flows back even into a large branch of the MMA (arrows in H). To reduce the volume of distribution, we glued the meningeal branch (arrows point to the cast in I), and the following injection of contrast medium achieves the choroidal blush (arrows in J).
Within group 3 (VP), in 2 different patients, chemotherapeutic agents were delivered through 2 different pathways within the same session (intraprocedural variability). However, even within group 2 (FPEC), the pattern could be somewhat variable, meaning that in some patients, we had to perform IAC through different branches of the ECA. For instance, in 1 case, we used 3 different anastomotic pathways (2 sessions through MMA, 1 session via the facial artery, and 3 sessions through the anterior deep temporal artery).
Particular Angiographic Procedures
In 14 patients, the angiographic procedure required a more creative approach. For instance, sometimes the stemming of the OA from the ICA was unfavorable (too angulated) and its direct catheterization was not possible. Thus, in 16 patients (16.2%), we had to customize the tip of the microcatheter, as previously reported,8 shaping it like an S to properly fit the anatomy of the patient (Fig 2A).
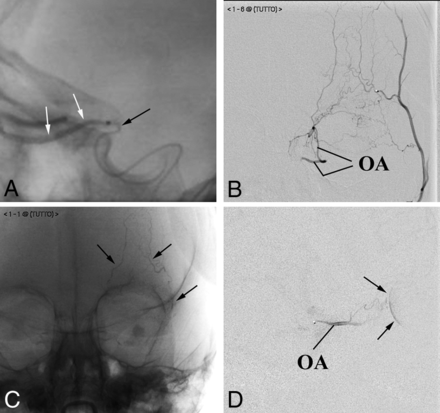
Particular angiographic procedures. A, Customization of the microcatheter. The tip of the microcatheter (black arrow), manually bent to fit the angioanatomy of the patient, has been firmly placed at the entrance of the OA to release the contrast medium (white arrows). B–D, Flow anterograde redirection within the OA. Anteroposterior projections. Contrast medium injection into the superficial temporal artery shows a rich network of small vessels connecting the superficial temporal artery with the OA (B). Embolization of the arterial network. The cast of glue outlines the embolized frontal vessels (arrows in C). The flow in the OA, redirected anterogradely (D), allows achieving the choroidal blush (arrows in D).
In other cases, 2 different hemodynamic conditions prompted us to embolize selected branches of the ECA: In 2 patients, we observed a too large amount of chemotherapy dispersion into branches of the MMA. To maximize drug availability to the target, we embolized a major branch of the MMA (Fig 1) as previously reported in other vascular areas.14 In the remaining patient (Fig 2B–D), the orbit not supplied through a single anastomosis that could be exploited for drug delivery and received blood mainly through an extensive network of several small branches connecting the superficial temporal artery with the supratrochlear artery. In this case, we navigated the superficial temporal artery and embolized the anastomotic network to induce the anterograde redirection of the blood flow within the ophthalmic artery (Fig 2D).
Outcome
The outcome of the eyes treated via the different patterns of drug delivery after a follow-up of at least 6 months is summarized in Table 1. The rate of success (tumor remission versus recurrence/enucleation) of the IAC was 63% for the eyes treated with an FPO, 77.7% for the eyes treated with an FPEC, and 47% for the eyes treated with a VP. No significant association was found between the pathway of drug delivery and the outcome of the treatment (P = .276).
Outcome of the eyes according to the pattern of IAC drug delivery
Data on the ophthalmologic response arranged according to the pattern of drug delivery are summarized in Table 2. A high response was achieved in 90.4%, 77.8%, and 88.2% of the treatments delivered, respectively, through an FPO, FPEC, and VP. A medium response was obtained in 4.1%, 0%, and 5.9% of the treatments delivered, respectively, through an FPO, FPEC, and VP; and a low response was achieved in 5.5%, 22.2%, and 5.9% of the treatments via an FPO, FPEC, and VP. Statistical analysis suggested that different patterns of drug delivery do not result in a significant change in the ophthalmologic response (P = .424).
Ophthalmologic response according to the pattern of IAC drug delivery
Adverse Events
A list of local adverse events observed in our survey is reported in Table 3. We compared the frequency of the more common transient local adverse events (palpebral edema/hyperemia, frontal edema/hyperemia, and retinal bleeding) to see if they could be preferentially associated with a particular pattern of IAC drug delivery (Table 3). We did not find any significant association between transient local adverse events and patterns of administration (P = .784). The number of events observed for all other temporary local adverse events was too low to attempt any statistical evaluation.
Local adverse events according to the pattern of IAC drug delivery
The same type of analysis was performed to compare the most frequent permanent local adverse events (chorioretinic atrophy, exotropia). Even in this case, permanent ocular complications could not be associated with any particular pattern of drug delivery (P = .233).
Permanent systemic adverse events have not been observed. In contrast, transient systemic adverse events, though rare, were found in 3 patients. They included blood disorders like febrile neutropenia (1 patient), treated with granulocyte colony stimulating factor; and bone marrow hypocellularity (2 patients). One of these patients presented with anemia that required blood transfusion. Two cases belonged to the group of patients characterized by an FPO of drug delivery, and 1 case, to the group of patients treated through an FPEC of drug delivery.
Intraprocedural Complications
A transient increase of airway resistance followed by blood oxygen desaturation of variable degrees from moderate to severe was observed in 200/448 procedures, resulting in a saturation of peripheral oxygen lower than 90% in 145 patients (32.3% of sessions) and lower than 60% in 5 patients. The latter 5 cases were accompanied by bradycardia and hypotension, leading to treatment interruption in 1 occasion. The above-mentioned cardiac and respiratory complications occurred following the insertion of the microcatheter into the cavernous segment of the ICA or during cannulation of the OA. A distal small embolism of the middle cerebral artery occurred in 2 patients, both treated via the ICA. One of them required 1-day monitoring in a neurointensive care unit under IV heparin infusion. All previously reported complications were transitory, and no child presented with clinical disabilities on reawakening.
Radiation Exposure
The radiation exposure was measured in a sample of 5 patients randomly selected for each group. For the PFO group, the dose-area product was 4.15 ± 0.83 Gy/cm2; it was 12.74 ± 2.34 Gy/cm2 for the PFEC group and 17.35 ± 3.47 Gy/cm2 for the VP group. Radiation exposure comparison among the groups was significantly different (P < .001).
Discussion
IAC in the treatment of intraocular retinoblastoma is currently a widely accepted therapeutic strategy used in specialized centers. Theoretically, the technique of drug delivery into the OA to target the tumor is not difficult if the neurovascular interventionalist has been properly trained. Indeed, navigation of the ICA and catheterization of the OA are facilitated by the very small size of the currently available microcatheters. However, in a remarkable number of cases, the procedure is more difficult and laborious than expected and cannot proceed conventionally. In such cases, the neurovascular interventionalist is required to use a more adaptable approach to minimize technical failures and to maximize IAC efficacy.
Two technically challenging scenarios potentially undermining IAC success can be found with a certain frequency in the angiographic room. The first scenario is a troublesome and unstable catheterization of the OA ostium despite its visualization by selective angiography of the ICA. As previously reported,9 this situation is usually secondary to a too angulated origin of the OA from the ICA, which makes the catheterization unsteady. In such cases, resuming the balloon technique applied to the ICA has been suggested9 despite all its drawbacks (ie, the risk of evoking ischemic complications, dilution of the drugs in a volume of blood that is too large, the reduced time of drug perfusion). We have found that this technical problem can be overcome by just customizing the tip of the catheter; in particular, it is possible to bend it to the desired angulation to fit the anatomy of the patient. Thus, >16% of our patients have been regularly treated as scheduled without the necessity of performing the balloon-assisted occlusion of the ICA.
The second unfavorable scenario is when an adequate choroidal blush is unattainable even when OA catheterization is achieved. In this situation, the contrast medium flows back into the ICA instead of diffusing along the OA without any visible vasospam. Indeed, the reflux of contrast medium into the ICA is likely secondary to an inversion of the blood flow within the OA due to several possible anastomoses with the ECA, which may provide alternative pathways for the orbital blood supply.12,15 In such cases, balloon occlusion of the ICA with drug infusion just below the block may not be as effective as the use of alternative routes through the ECA because the blood supply to the orbit comes just from the latter source. In children, the hemodynamic balance between the OA and the ECA may be subtle. This balance is verified by the number of eyes treated with a VP (17.2% of cases) and, when the pattern was FPEC, by the various anastomoses that, in some cases, have been used for drug delivery in the same children.
Actually, even when previous visualization of the OA has been successful, subsequent attempts to achieve an acceptable choroidal blush through the OA in the same child may fail due to momentary imbalances of the local hemodynamics in favor of the ECA, leading to an inversion of the flow. A series of unpredictable and possibly unknown factors, including vasospasm, are possibly at the basis of such phenomenona. The presence of the catheter itself into the OA origin has been suggested as a possible cause of hemodynamic disturbance leading to the activation of collateral circulations.10 Previous chemotherapy may even reduce the caliber of the OA,4 inducing the recruitment of blood via anastomoses with the ECA. At any rate, the presence of anastomoses between the OA and the ECA has been suspected as a variable that could negatively impact the effectiveness of the IAC.10 Far from it, we believe that the ECA and some of its branches represent potential alternative routes for IAC. In our and in others' experience,9,16,17 some of these routes have already been used to deliver antitumoral drugs. Our study shows that 16.29% of procedures occurred via 1 of the many alternative pathways previously described and involving branches of the ECA.12 This adaptable approach allowed us to increase the number of successful sessions of IAC. In particular, we calculated that 24.52% of eyes could have skipped at least 1 session of IAC if we had not looked for anastomotic pathways. In a retrospective review of 351 procedures of IAC, Klufas et al9 reported that 7.8% of IAC infusions occurred through the MMA and that in 4.7% of cases, they resumed the balloon-assisted occlusion of the ICA because a suitable anastomosis between the OA and MMA was not found.
By using other anastomotic pathways, we show that IAC can be successfully performed in a larger number of patients. Thus, a promising technique has been recently proposed to manage competitive backflow from ECA collaterals. Instead of searching and catheterizing the anastomoses between the ECA and the OA, anterograde redirection of the blood flow was achieved by balloon occlusion of the ECA.18 However, even this approach has drawbacks (puncture of both femoral arteries, 2 catheter-microcatheter systems within the same artery), and its efficacy/safety versus catheterization of the ECA branches still requires validation.
Although the use of the ECA and its branches for IAC has been previously reported,9,16,17 its effectiveness and safety have never been demonstrated in comparison with regular IAC via the OA. Our retrospective study suggests that the use of branches of the ECA in selected cases is as effective as the direct injection into the OA in terms of ophthalmologic response (90.4% for PFO, 88.2% for VP, 77.8% for PFEC). Concerning the outcome, our results show that no statistically significant difference can be found when comparing the 3 groups. However, in light of the limited number of drug deliveries through branches of the ECA and the short follow-up, results have to be interpreted with caution and conclusions should be validated in a larger population and in a longer time perspective.
An even more adaptable approach for IAC can be applied in extremely selected cases. For instance, we artificially altered the orbital circulation, gluing some extraorbital vessels to reduce the volume of drug distribution or to create a more favorable intraorbital circulation to target the tumor. Although the desired goal was achieved and the choroidal blush was obtained, the number of cases was too low to allow a valid statistical evaluation on the efficacy of such procedures. We are aware that embolization of ECA branches is potentially dangerous even in very experienced hands because it can induce retinal or cranial nerve ischemia or stroke due to ICA reflux. However, in our case, embolizations have been performed by a skilled interventional neuroradiologist (S.B.) with experience in managing glue for arteriovenous malformations or fistulas even in the pediatric age group. In addition, this type of embolization is performed with a very small amount of glue for a proximal deafferentation.
Data concerning transient adverse events are in line with those described in other studies.6,19⇓–21 Procedural complications, including embolic events, are rarely described in the literature,21 and this finding is consistent with our experience (0.4% of sessions). On the other hand, cardiac and respiratory reactions are commonly observed during this procedure. The pathophysiologic mechanism of these complications is currently unknown, likely involving an autonomic reflex.22 Most interesting, neither the cardiac and respiratory complications nor the embolic events ever occurred when patients were treated through the ECA; this finding suggests that this route of drug delivery, though more troublesome, should be considered safer than the OA cannulation.
Systemic adverse events, mainly pancytopenia, are also rare, probably due to the cumulative result from previous administration of systemic chemotherapy. Most local adverse events were transient and minor. Permanent local adverse events (chorioretinic atrophy and exotropia) were observed in 18.9% of eyes, a lower incidence compared with other reports.3,23 Statistical analysis did not allow us to associate any adverse event with particular patterns of drug delivery. Concerns remain regarding the toxic effects of irradiation from repeat fluoroscopy in children with retinoblastoma. However, although the VP and PFEC showed higher exposure levels compared with the FPO, the overall irradiation was far lower than the toxic levels previously reported.8
Conclusions
IAC is a valuable therapeutic strategy to treat intraocular retinoblastoma. However, in some cases, it may present technical challenges requiring an unconventional approach, in particular concerning the search for alternative routes of drug delivery through branches of the ECA. The use of these pathways is as effective as the traditional drug infusion through the OA in terms of ophthalmologic response without increasing adverse events and complications.
Footnotes
E. Bertelli and S. Leonini contributed equally to this study.
REFERENCES
- Received September 23, 2015.
- Accepted after revision December 9, 2015.
- © 2016 by American Journal of Neuroradiology