Abstract
BACKGROUND AND PURPOSE: Medullary thyroid carcinoma is an uncommon malignancy that is challenging to diagnose. Our aim was to present our experience using core needle biopsy for the diagnosis of medullary thyroid carcinoma compared with fine-needle aspiration.
MATERIALS AND METHODS: Between January 2000 and March 2012, 202 thyroid nodules in 191 patients were diagnosed as medullary thyroid cancer by using sonography-guided fine-needle aspiration, core needle biopsy, or surgery. One hundred eighty-three thyroid nodules in 172 patients were included on the basis of the final diagnosis. We evaluated the sensitivity and positive predictive value of fine-needle aspiration and core needle biopsy for the diagnosis of medullary thyroid cancer. We compared the rate of a delayed diagnosis, a diagnostic surgery, and surgery with an incorrect diagnosis for fine-needle aspiration and core needle biopsy and investigated the factors related to the fine-needle aspiration misdiagnosis of medullary thyroid cancer.
RESULTS: Fine-needle aspiration showed 43.8% sensitivity and 85.1% positive predictive value for the diagnosis of medullary thyroid cancer; 25.7% (44/171) of patients had a delayed diagnosis, while 18.7% (32/171) underwent an operation for accurate diagnosis, and 20.5% (35/171) underwent an operation with an incorrect diagnosis. Core needle biopsy achieved 100% sensitivity and positive predictive value without a delay in diagnosis (0/22), the need for a diagnostic operation (0/22), or an operation for an incorrect diagnosis (0/22). A calcitonin level of <100 pg/mL was the only significant factor for predicting the fine-needle aspiration misdiagnosis of medullary thyroid cancer (P = .034).
CONCLUSIONS: Core needle biopsy showed a superior sensitivity and positive predictive value to fine-needle aspiration and could optimize the surgical management in patients with medullary thyroid cancer. Because the ability of fine-needle aspiration to diagnose medullary thyroid cancer significantly decreases in patients with serum calcitonin levels of <100 pg/mL, core needle biopsy could be indicated for these patients to optimize their surgical management.
ABBREVIATIONS:
- AUS
- atypia of undetermined significance or follicular lesion of undetermined significance
- CNB
- core needle biopsy
- FNA
- fine-needle aspiration
- MTC
- medullary thyroid carcinoma
- PTC
- papillary thyroid carcinoma
- US
- ultrasound
Medullary thyroid carcinoma (MTC) is an uncommon malignancy that is challenging to diagnose. Although it accounts for only 3%–5% of thyroid cancer diagnoses, it causes 15% of thyroid cancer–related deaths due to its aggressiveness.1 Due to a lack of good treatment options other than surgery, early diagnosis of MTC and complete surgical resection comprising at least total thyroidectomy with central lymph node dissection offers the best chance for a cure.2⇓⇓–5 In that respect, accurate and timely diagnosis of MTC is essential to ensure the appropriate surgical procedure.6⇓⇓–9
An early presurgical diagnosis of MTC remains a diagnostic challenge in clinical practice, however. Although fine-needle aspiration (FNA) cytology is an important diagnostic tool for evaluating thyroid nodules, its low sensitivity for diagnosing MTC limits an optimal preoperative evaluation and an operation in approximately half of the patients.10,11 Serum calcitonin level measurement in patients with thyroid nodules is a sensitive and specific marker with even better diagnostic accuracy than cytology in unsuspected MTC, though the routine measurement of serum calcitonin levels in nodular thyroid disease and the cutoff value for a diagnosis of MTC are still debatable.11⇓–13 Due to these diagnostic difficulties, some MTC is still incidentally discovered after a diagnostic operation or after an operation with an incorrect presurgical diagnosis—this situation presents the risk of an incomplete therapeutic approach and a less favorable prognosis.10,14,15 Therefore, early and accurate diagnosis of MTC is of crucial importance for optimal management.
We studied core needle biopsy (CNB) for the diagnosis of MTC at 3 medical institutions during a 10-year period. The study was designed to evaluate the following: 1) the diagnostic performance of CNB, 2) its impact on surgical management compared with FNA, and 3) factors related to the FNA misdiagnosis of MTC.
Materials and Methods
The institutional review boards approved this retrospective study at the 3 participating sites and required neither patient approval nor informed consent for review of the images and medical records. However, informed consent for FNA or CNB was obtained from all patients before biopsy.
Patients
This retrospective analysis was based on patient data collected from 3 medical institutions (ie, Asan Medical Center, Seoul National University Hospital, and the Human Medical Imaging and Intervention Center). We reviewed the medical records of patients who were diagnosed with MTC between January 2000 and March 2012 at the 3 institutions. During this time, 202 thyroid nodules in 191 patients were initially diagnosed as MTC by using 1 of the following methods: ultrasound (US)-guided FNA, US-guided CNB, or surgery. Of these 191 patients, 19 were excluded because they were surgically treated at another hospital (n = 5) or were without FNA or CNB results before the operation (n = 14). This study finally included 183 thyroid nodules in 172 patients (112 women and 60 men; mean age, 49.6 years; range, 17–91 years).
Of the 183 nodules in 172 patients, FNA was initially used in 182 nodules in 171 patients. CNB was used in 22 nodules in 21 patients as the initial approach (n = 1), after the FNA results of non-MTC (n = 13), or simultaneously with FNA (n = 8). The final diagnosis was based on the pathology results obtained after surgery for all nodules except for 2 in 2 inoperable patients with high calcitonin levels of 1330 and 82,900, respectively.
US-Guided FNA and CNB Procedures
US examinations were performed by using 1 of 4 US systems: an iU22 U (Philips Healthcare, Best, the Netherlands), an EUB-7500 U (Hitachi Medical Systems, Tokyo, Japan), an Aplio XG (Toshiba Medical Systems, Tokyo, Japan), or an HDI 5000 (Philips Healthcare), equipped with a linear, high-frequency probe (5–14 MHz). All US examinations and US-guided FNA or CNB procedures were performed by 4 clinically experienced thyroid radiologists with 12–19 years of thyroid US experience or by residents and fellows under their supervision.
US-guided FNAs were performed with a combination of 25-, 23-, and 21-ga needles and a combination of capillary and aspiration FNA techniques according to the characteristics of the nodules. Each lesion was aspirated at least twice (range, 2–4 times). Materials obtained from the FNA were immediately placed in 95% alcohol for Papanicolaou staining.
US-guided CNBs were performed by using a disposable, 18-ga, double-action, spring-activated needle (1.1- or 1.6-cm excursion) (TSK Acecut; Create Medic, Yokohama, Japan) after local anesthesia with 1% lidocaine. Before the needle insertion, vessels along the approach route were carefully evaluated by power Doppler US to prevent procedure-related hemorrhage. Using a freehand technique, we advanced the core needle from the isthmus of the thyroid toward the target nodule by using the transisthmic approach. When the needle tip was advanced into the edge of the nodule, the stylet and cutting cannula of the needle were sequentially, carefully fired. The number of tissue cores obtained by CNB ranged from 1 to 3. A second or third CNB was performed when a lesion was considered inaccurately targeted, as in the case of small nodules or when an adequate tissue core was not obtained by visual inspection. Each patient was observed after firm, local compression of the biopsy site for 10–20 minutes after FNA or CNB. If a patient had pain or neck swelling, a repeat US examination was performed to evaluate possible complications.16
Cytologic and Histopathologic Analyses
The FNA, CNB, and surgical specimens were reviewed by experienced cytopathologists with 8–10 years of clinical experience in thyroid cytopathology; real-time cytology was not available during the biopsy procedure. FNA cytology diagnoses were categorized as nondiagnostic, benign, atypia of undetermined significance or follicular lesion of undetermined significance (AUS), follicular neoplasm/suspicious for follicular neoplasm, suspicious for malignancy, or malignant according to the Bethesda System for Reporting Thyroid Cytopathology.17 Because the diagnostic criteria of CNB have not been standardized for thyroid nodules, the CNB histologic diagnoses were categorized into the same categories as those in the Bethesda System.18⇓–20 For evaluating the specific diagnostic performance for MTC on both the FNA and CNB, the “suspicious for malignancy” or “malignancy” reading was further categorized into subtypes suggesting papillary thyroid carcinoma (PTC), MTC, or other malignancy.
Additional special staining was performed on a case-by-case basis according to the cytopathologists' preferences and concerns. Calcitonin staining on FNA or CNB specimens was not routinely used for these readings, though it could be used as an additional requirement of the cytopathologists when the FNA or CNB findings were suspicious for MTC, to obtain a confirming diagnosis.
Statistical Analysis
Statistical analysis was performed by using the SPSS software package (Version 19.0 for Windows; IBM, Armonk, New York). Categoric data were summarized by using frequencies and percentages. The diagnostic performance of FNA and CNB for MTC was evaluated by using the sensitivity and positive predictive value. The χ2 test, Student t test, and Mann-Whitney U test were used to evaluate the factors related to the FNA misdiagnosis of MTC. FNA misdiagnosis was defined as any FNA cytologic diagnosis except MTC. Binary logistic regression was used for multivariate analysis. The Spearman rank correlation was used to evaluate the relationship between the size and the calcitonin level. A P value < .05 was considered statistically significant.
To assess the clinical impact of FNA or CNB on the surgical management of MTC in current practice, we evaluated the rate of the delayed diagnosis, diagnostic surgery, and surgery by using an incorrect presurgical diagnosis as follows: “Delayed diagnosis” was defined as a nodule initially misdiagnosed as non-MTC but finally confirmed as MTC on the next FNA or CNB. “Diagnostic surgery” was defined as a nodule initially misdiagnosed as non-MTC (ie, nondiagnostic, benign, or AUS) but confirmed as MTC on subsequent surgery performed for a diagnostic purpose. “Surgery with an incorrect diagnosis” was defined as 1 of the following 2 categories: First, the nodule was initially misdiagnosed as non-MTC (ie, a follicular neoplasm/suspicious for follicular neoplasm, suspicious for or a definite diagnosis of PTC, or other types of malignancy) but was finally confirmed as MTC following the operation. Second, the nodule was initially misdiagnosed as MTC but was finally confirmed as non-MTC following the operation.
Results
Demographic Data
Among the 183 nodules in 172 patients, 170 nodules in 159 patients were confirmed as MTCs and 13 nodules in 13 patients were confirmed as non-MTCs following surgery. The mean nodule size was 20.3 ± 15.7 mm (range, 3.0–85.0 mm). Of 170 confirmed MTCs, 26 nodules in 20 patients were hereditary MTCs and 144 nodules in 139 patients were sporadic MTCs.
The serum calcitonin level was measured before surgery in 148 patients with 159 nodules. The median serum calcitonin level was 389.0 pg/mL (range, 1.5–82,900.0 pg/mL), with a subclassification of ≥100 pg/mL (n = 92), ≥10 and <100 pg/mL (n = 41), or <10 pg/mL (n = 15).
Diagnostic Performance of FNA or CNB
Table 1 shows the comparison of the initial FNA and CNB results with the final pathology results. The sensitivity and positive predictive value for a diagnosis of MTC were 43.8% (74/169) and 85.1% (74/87), respectively, following the initial FNA, though they were both 100% (22/22) after CNB. MTCs were commonly misinterpreted as AUS (44/169, 26.0%) or other types of malignancy (15/169, 8.9%) after FNA. Thirteen false-positively diagnosed MTCs were actually PTC (7/13, 53.8%) follicular adenoma or carcinoma (4/13, 30.8%), hyalinizing trabecular tumor (1/13, 7.7%), or anaplastic carcinoma (1/13, 7.7%).
Comparison of the initial FNA and CNB results with the final pathology resultsa
Table 2 shows the relationship between the serum calcitonin level and the diagnosis of MTC after FNA or CNB procedures. In patients with a serum calcitonin level of <10 pg/mL, 10 of 11 nodules with an FNA diagnosis of MTC were confirmed as non-MTC following the surgery. However, in this range, 5 nodules were finally confirmed as MTCs after surgery even though 4 were not diagnosed after FNA. One nodule with a CNB diagnosis of MTC was correct after surgery in this range.
Relationship of the serum calcitonin level and the diagnosis of MTC after FNA or CNB proceduresa
Clinical Impact of FNA or CNB on Surgical Management
Delayed Diagnosis.
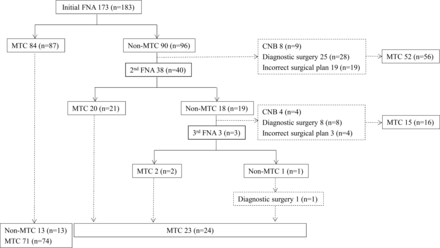
Figure 1 shows the diagnostic flowchart for the patients following FNA. A delayed diagnosis was noted in 25.7% (44/171) of patients after FNA, while there was none in patients (0/22) after CNB. A delayed diagnosis continued in 22.2% (8/36) of these patients following the second FNA. Therefore, the delayed diagnosis rate differed significantly between FNA and CNB (P = .007) (Table 3).
Diagnostic flowchart of patients following the initial FNA. Data indicate the number of patients, with the number of nodules in parentheses.
Comparison of the FNA and CNB results with the factors regarding surgical decision-making of MTCa
Diagnostic Surgery.
Diagnostic surgery was performed in 18.7% (32/171) of the patients after FNA, though it was not performed in any patients (0/22) after CNB. Of 32 patients, 24 with 28 nodules underwent diagnostic surgery after having initial FNA results of nondiagnostic (n = 2), benign (n = 7), and AUS (n = 19), and 7 patients with 7 nodules underwent diagnostic surgery after having second FNA results of nondiagnostic (n = 1) and AUS (n = 6). One patient underwent diagnostic surgery after 3 repetitive FNA results of AUS. The diagnostic surgery rate was significantly different between FNA and CNB (P = .028) (Table 3).
Surgery with an Incorrect Diagnosis.
Surgery with an incorrect diagnosis was performed in 20.5% (35/171) of the patients after FNA, though it was not performed in any patients (0/22) after CNB. There were 2 types of surgery with an incorrect diagnosis: The first was surgery with a false-negative diagnosis of MTC; 12.9% (22/171) of the patients underwent surgery with a cytologic diagnosis of follicular neoplasm/suspicious for follicular neoplasm (n = 10), PTC (n = 4), or other types of malignancy (n = 8). The second was surgery with a false-positive diagnosis of MTC; 7.6% (13/171) of the patients underwent surgery with a cytologic diagnosis of MTC, which was, however, finally determined after surgery to be PTC (n = 7), follicular adenoma (n = 2), follicular carcinoma (n = 2), hyalinizing trabecular tumor (n = 1), or anaplastic carcinoma (n = 1). The rate of surgery with an incorrect diagnosis differed significantly between FNA and CNB (P = .016) (Table 3).
Factor Analysis Related to the FNA Misdiagnosis of MTC
Table 4 shows the factors related to the FNA misdiagnosis of MTC. On bivariate analyses, a serum calcitonin level of <100 pg/mL was the only significant factor for predicting the FNA misdiagnosis of MTC (P = .034). Mass size was not a significant factor (P = .258) despite the positive correlation with the serum calcitonin level (ρ = 0.605). A binary logistic regression analysis was performed to determine the independent factors for predicting the FNA misdiagnosis of MTC. A serum calcitonin level of <100 pg/mL showed a significant association with the FNA misdiagnosis (P = .036) (95% CI, 1.052–4.355; odds ratio, 2.141).
Factor analysis related to the FNA misdiagnosis of MTCa
Complications
There were no complications leading to substantial morbidity or disability or resulting in a hospitalization after FNA or CNB. One patient after FNA had neck discomfort due to thyroid parenchymal edema. However, there were no major complications after FNA or CNB.
Discussion
Our study confirms that FNA cytology has 43.8% sensitivity and 85.1% positive predictive value for the diagnosis of MTC, while the respective values for CNB were both 100.0%. FNA cytology caused a delayed diagnosis in 25.7% (44/171), required diagnostic surgery in 18.7% (32/171), and led to surgery with an incorrect diagnosis in 20.5% (35/171) of the study patients. Serum calcitonin levels of <100 pg/mL were the only significant factor related to the FNA misdiagnosis of MTC.
FNA cytology for a diagnosis of MTC has been proved to have a low sensitivity of 43.7% in recent multicenter and international studies.10 It has been reported with a wide range from 30% to 89% in smaller series.11,13,21,22 Its low sensitivity leads to the misdiagnosis of MTC and negatively impacts patient management in several ways. A delayed diagnosis can alter the MTC stage at the time of surgery and may thus influence both the surgical extent and the patient prognosis.6⇓⇓–9 Diagnostic surgery or surgery with an incorrect diagnosis can overlook the evaluation for multiple endocrine neoplasia syndrome before surgery and can also be responsible for suboptimal surgery, which may require additional surgery for completion and may carry an increased risk of morbidity.6⇓⇓–9 In our study, the low sensitivity of FNA for MTC was reconfirmed in 172 patients at 3 medical institutions during a 10-year period; 56.2% of the patients with MTC were initially misdiagnosed; therefore, proper surgical management was delayed or suboptimal after FNA.
Among the available diagnostic tools for MTC, serum calcitonin level measurement has been the most commonly used due to its higher sensitivity for MTC than FNA cytology.11⇓–13 Because the appropriate use of serum calcitonin level measurement is helpful for detecting unsuspected MTC, it is commonly used in patients with thyroid nodules to prevent the risk of a false-negative cytologic diagnosis. Patients with a serum calcitonin level of ≥100 pg/mL are strongly recommended for surgery, even though the cytologic diagnosis is not fulfilled for MTC under the current guidelines.2,5,14,15 However, there is a diagnostic gray zone in patients with a serum calcitonin level between 10 and 100 pg/mL, and the cutoff value for a diagnosis of MTC is still debatable.14 Our study results also showed 2 diagnostic dilemmas associated with the serum calcitonin level. First, MTC could not be completely excluded even in the normal range of serum calcitonin levels. Although most presurgical diagnoses of MTC after FNA were false-positive in this range, 5 nodules were proved to be MTCs following surgery. Second, a serum calcitonin level of <100 pg/mL, a diagnostic gray zone in the clinical setting, was also an independent factor related to the FNA misdiagnosis of MTC. Therefore, in these patients, CNB may have been a useful diagnostic tool for an accurate diagnosis and proper management of MTC in our study.
The diagnostic role of CNB for thyroid nodules is currently being investigated. Because US-guided CNB using a spring-activated biopsy needle has been reported safe and effective by Quinn et al,23 several investigators have demonstrated the usefulness of CNB in diagnosing thyroid nodules with 90.3% (range, 78.5%–100%) sensitivity and 98.4% (range, 87%–100%) positive predictive value for thyroid malignancy.18⇓–20,24⇓–26 Currently, the role of CNB has focused on the diagnosis of nodules with previously nondiagnostic results on FNA and, therefore, on preventing unnecessary diagnostic surgery.19,24,25,27 For the diagnosis of a specific type of malignancy, however, CNB has also showed the possibility of superior diagnostic performance to that of FNA for thyroid lymphoma, anaplastic carcinoma, and metastasis in a small series of studies.26,28⇓⇓–31 The complication rate was reported to be <1% and was considered to be similar to that of FNA if an experienced clinician performed the procedure.18,19 In our study, all 22 patients who underwent CNB were correctly diagnosed with MTC without any major complications. Of these patients, the serum calcitonin levels were <100 pg/mL in 8 patients (36.4%), but the CNB diagnoses were consistent for MTC. CNB showed a precise histologic diagnosis in all cases regardless of the serum calcitonin levels.
Our study has several limitations. First, its retrospective design may have introduced a selection bias. Because the low incidence of MTC limits prospective study design, however, it may be the best method for retrospectively reviewing the clinical experience during long periods at many medical institutions. Second, FNA results before 2010 were re-interpreted according to the Bethesda System. Third, because the diagnostic categories of CNB specimens have not yet been standardized, this aspect requires further research. Fourth, a calcium stimulating test or calcitonin level measurement of aspiration needle washout fluids or both were not performed in our study. However, our experience using CNB provides useful information as a complementary diagnostic tool for MTC. Further studies are required for deciding a proper indication of each technique. Fifth, there is significant difference in size of the FNA and CNB group. Further studies are required.
Conclusions
A relatively small sample of CNBs showed a superior sensitivity and positive predictive value to those of FNA and could thus optimize the surgical management in patients with MTC. Because the FNA diagnosis of MTC significantly decreases in patients with serum calcitonin levels of <100 pg/mL, CNB could be a complementary diagnostic tool for these patients to optimize surgical management.
References
- Received June 18, 2014.
- Accepted after revision January 4, 2015.
- © 2015 by American Journal of Neuroradiology