Abstract
BACKGROUND AND PURPOSE: Infarct volume may predict clinical outcome in acute stroke, but manual segmentation techniques limit its routine use. We hypothesized that computer-assisted volumetric analysis to quantify acute infarct volume will show no difference compared with manual segmentation but will show increased speed of performance and will correlate with outcome.
MATERIALS AND METHODS: Patients with acute stroke younger than 18 years were included. Infarct volume on diffusion-weighted imaging was quantified by using computer-assisted volumetric and manual techniques. The Pediatric Stroke Outcome Measure scored clinical outcome. Computer-assisted volumetric and manual techniques were compared with correlation coefficients. Linear regression analysis compared Pediatric Stroke Outcome Measure with core infarct volume and percentage volume of brain infarction.
RESULTS: Twenty-three patients were analyzed (mean age, 4.6 years). Mean infarct volume from computer-assisted volumetric and manual approaches was 65.6 and 63.7 mL, respectively (P = .56). Concordance correlation between methods was 0.980, and between users, 0.968. The mean times for segmentation between computer-assisted volumetric and manual techniques were <1 minute and 7.3 minutes (P < .001). The mean infarct volumes for good and poor outcome groups were 7.4 and 75.7 mL (P < .007). The mean percentages of infarcted brain parenchyma for good and poor outcome groups were 0.6% and 10.4% (P < .006). Volumes of 32 mL and 3% for infarcted brain were associated with poor outcome in all patients.
CONCLUSIONS: Computer-assisted volumetric quantification of infarct volume is reproducible, is significantly faster than manual techniques, and may have important applications for future clinical workflow. Core infarct volumes and infarct percentage correlated with outcome severity.
ABBREVIATIONS:
- CAV
- computer-assisted volumetry
- PSOM
- Pediatric Stroke Outcome Measure
Stroke in the pediatric population is occurring at increasingly younger ages with an increasing incidence estimated at 3–5 per 100,000 according to the US Nationwide Inpatient Sample (http://www.healthdata.gov/data/dataset/hcup-nationwide-inpatient-sample-nis), which showed annual increases in acute ischemic stroke admissions from 1995 to 2008.1⇓⇓⇓⇓–6 However, pediatric stroke remains under-recognized among health care providers; a lack of evidence-based treatment and management guidelines specific to the pediatric population further complicates this problem.7,8 This represents a critical health care problem, given the potential cost to society in terms of life-years of disability and life-years lost in the face of increasing incidence of acute ischemic arterial infarction of children.6,7,9,10
Recent studies support infarct volume quantification as a potential tool in the pediatric population for predicting clinical outcome.11⇓–13 For example, Ganesan et al14 observed that infarcts of >10% parenchymal volume on T2-weighted imaging were associated with poor outcomes. Additionally, Domi et al15 reported that reduced diffusion in the corticospinal tract was a predictor of motor outcomes. Furthermore, investigations in adults have suggested that core infarct volume quantification correlates best with long-term outcome; this finding lends credibility to a similar approach in pediatric stroke.16⇓⇓–19
Currently, the criterion standard for volumetric assessment involves manual segmentation, which can be time-consuming and technically challenging. These impediments may limit its use in the emergency setting, where time is critical for management. For these reasons, computer-assisted volumetry (CAV) applied to diffusion-weighted imaging may represent a potential tool to aid in the detection of core infarct volume in the pediatric population. With regard to neuroimaging, CAV has recently been used in the examination of recurrent glioblastoma with high reproducibility and speed compared with conventional manual approaches.
The purpose of this study was to describe a novel CAV technique for assessment of core infarct volume within the pediatric population. Specifically, we describe the reliability and feasibility of this technique compared with traditional manual approaches in patients with acute stroke. Additionally, the relationship between infarct volume and clinical outcomes by using the Pediatric Stroke Outcome Measure (PSOM) scale will be obtained.20,21
Materials And Methods
Subjects
After institutional review board approval of this Health Insurance Portability and Accountability Act–compliant retrospective study, a query of the neuroradiology department data base for pediatric patients with acute arterial ischemic infarct at our institution from January 2011 to November 2012 was conducted. Acute arterial ischemic infarct was defined as an acute neurologic deficit in a patient with an MR imaging abnormality on DWI consistent with infarction and corresponding hypointense signal on the apparent diffusion coefficient map. Inclusion criteria were pediatric patients defined as younger than 18 years of age. Imaging inclusion criteria were MR imaging with DWI performed within 72 hours of admission to the hospital. Exclusion criteria were hypoxic-ischemic encephalopathy, intraparenchymal hemorrhage, prior infarcts, incomplete imaging, or poor image quality (motion degradation) and incomplete follow-up. Additionally, patients without medical records sufficient to obtain PSOM scores were also excluded.
Imaging Techniques
All imaging was performed on a 1.5T MR imaging system (Signa; GE Healthcare, Milwaukee, Wisconsin) with an 8-channel head-array coil (Signa HDxt; GE Healthcare). We performed the following sequences: sagittal T1-weighted (TR, 450 ms/TE, 6.5 ms; 5-mm thickness; echo-train length, 4; FOV, 24 cm; 256 × 224 matrix); axial T1-weighted (TR, 433 ms/TE 6.5 ms; 5-mm thickness; echo-train length, 4; FOV, 24 cm; 256 × 224 matrix); axial T2-weighted (TR, 5000 ms/TE, 90 ms; 5-mm thickness; echo-train length, 28; FOV, 24 cm; 320 × 320 matrix); and axial FLAIR imaging (TR, 8800 ms/TE, 120 ms; TI, 1650 ms; echo-train length, 1.0; 5-mm thickness; FOV, 24 cm; 256 × 192 matrix). Axial diffusion-weighted imaging (TR, 8000 ms/TE 88 ms; echo-train length, 1.0; 5-mm thickness; 128 × 128 matrix; FOV, 24 cm) was performed by using 2 b-values (0 and 1000 s/mm2) and 3 diffusion-encoding gradient directions. Corresponding ADC maps were generated by commercial scanner software (FuncTool software; GE Healthcare).
Core Infarct Volume Segmentation
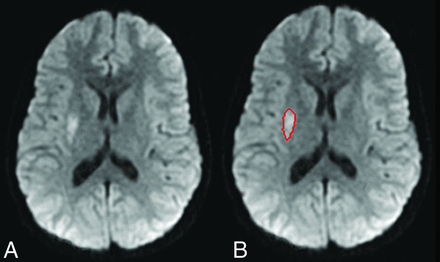
Two radiologists, including 1 with a Certificate of Added Qualification in neuroradiology, performed CAV analysis. The boundary of acute infarct was delineated on DWI sequences by using a proprietary segmentation algorithm developed in the Laboratory for Computational Image Analysis in the Department of Radiology, Columbia University Medical Center.22,23 The computer-assisted algorithm was originally developed for liver lesions and has since been adapted for different applications, including, most recently, the volume of enhancing tumor in glioblastoma multiforme.24⇓–26 For the first step of the volume measurement, the entire infarct volume was separated from surrounding anatomic structures by using a segmentation algorithm that combines the image analysis techniques of active contours and a level set approach. Once the segmentation was completed on an image, the infarct contour was propagated to its neighboring images, serving as an initial region of interest for subsequent segmentations on the neighboring images. This process was continued iteratively until all the infarct images were segmented. Once the segmentation was finalized, infarct volumes were automatically calculated (Fig 1). For brain volume, we determined the contour of the brain cortical surface from which ventricular volume is subtracted by contouring the ventricular lining.
A, Reduced diffusivity within the right putamen consistent with an area of acute infarction. B, The contour derived from the semiautomated computer segmentation software that is used to derive infarct volume.
The same 2 radiologists, both of whom were blinded to the patients' prior CAV volumes and clinical outcomes, performed manual segmentation of infarct volume. Segmentation was performed by manual tracing of regions of reduced diffusion on DWI sequences. All manual segmentations were performed at a dedicated workstation (Advantage Workstation, Version 4.3; GE Healthcare). To reduce bias from prior CAV analysis, we performed manual measurements 3 months after the initial CAV analysis. All measurements were recorded in milliliters, and the time required to perform CAV and manual segmentation for core infarct volume was noted.
Correlation with Clinical Outcomes
Clinical outcome was scored by using the PSOM, which uses neurologic evaluation to examine sensorimotor function bilaterally, productive and receptive language, and cognitive and behavioral development.20,21 The PSOM is typically scored as good (healthy or mild deficit) and poor (moderate or severe), and validity of the PSOM is not significantly affected when performed retrospectively.20 Clinical data and PSOM were determined from chart review by using the examination findings of a pediatric neurologist. All patients were evaluated by a neurology attending physician following stroke, which was confirmed through chart review. Two neuroradiologists performed chart review.
Statistical Analysis
Mean manual and semiautomated volumetric measurements and segmentation times were compared by using a paired t test. Concordance correlation coefficients with corresponding confidence intervals between semiautomated and manual volumetric measurements were obtained. For analysis of clinical outcomes, core infarct volumes and the percentage of infarcted parenchymal volumes were compared among patients with good and poor outcomes by using the Wilcoxon rank sum test. Linear regression analysis was performed comparing the PSOM with core infarct volume and percentage volume of brain infarction. All statistical analysis was performed by using MedCalc for Windows, Version 12.2.1.0 (MedCalc Software, Mariakerke, Belgium). For this study, a P value of < .05 was considered statistically significant.
Results
Subjects
In total, 29 patients were identified, of which 79.3% (23/29) met the inclusion criteria. Patient demographics are listed in Table 1. These included 12 male and 11 female patients (age range, 0–17.6 years; mean age, 4.6 years) presenting with acute stroke. Clinical follow-up ranged from 0.8 to 19.3 months (mean, 5.1 months). Six patients were excluded. One had hypoxic-ischemic encephalopathy rather than acute stroke, 1 had marked intraparenchymal hemorrhage and prior infarcts, 1 had incomplete imaging with poor image quality (motion degradation), and another had incomplete clinical follow-up. An additional 2 patients did not have all test items in their medical records to calculate a PSOM score and were excluded.
Demographics of patients
Comparison between Manual and CAV Analysis
The mean core infarct volumes obtained for CAV and manual approaches were 65.6 and 63.7 mL, respectively (P = .56). The mean total brain volume was 936.6 mL. The concordance correlation between the methods was 0.980 (95% CI, 0.956–0.991). The concordance correlation for CAV measurements between 2 users was 0.968 (95% CI, 0.935–0.985). The concordance correlation for manual segmentation between 2 users was 0.978 (95% CI, 0.964–0.989). The concordance for mild and large infarcts with CAV measurements was 0.999 (95% CI, 0.994–1.000) and 0.962 (95% CI, 0.915–0.983), respectively. The concordance for mild and large infarcts with manual measurements was 0.976 (95% CI, 0.943–0.990) and 0.976 (95% CI, 0.953–0.983), respectively.
The mean times for segmentation between computer-assisted and manual techniques (including times for opening and saving images) were <1 minute and 7.3 minutes, respectively (P < .01).
Correlation with Clinical Outcomes
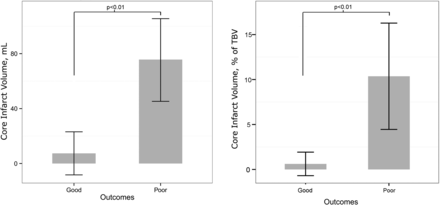
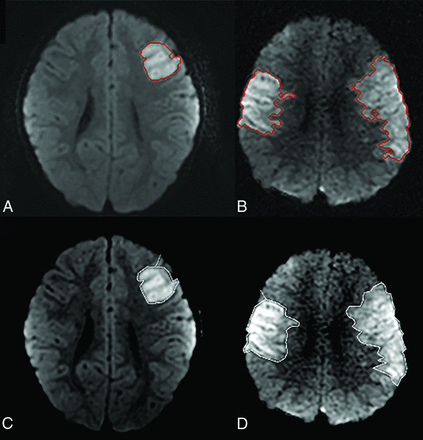
Patient outcomes and stroke territory involvement are listed in Table 2, and patient outcomes with respect to stroke characteristics, including corresponding confidence intervals, are listed in Table 3. With regard to outcomes, 78.3% (18/23) had poor outcomes, and 21.7% (5/23) had good outcomes by the PSOM scores. Mortality was observed in 21.7% (5/23) cases. Mean core infarct volumes for the good and poor outcome groups were 7.4 and 75.7 mL, respectively (P < .007). Mean percentages of infarcted brain parenchyma for the good and poor outcome groups were 0.6% and 10.4%, respectively (P < .006). There was a significant correlation between PSOM and infarct volume (P < .01) and percentage of brain parenchymal infarction (P < .04). This is displayed graphically in Fig 2 and summarized in Table 4. Core infarct volumes of >32 mL and percentage of infarcted brain parenchyma of >3% had poor outcomes in all cases. Examples of segmentation results from CAV and manual approaches for infarcts in poor and good outcome cohorts are provided in Fig 3.
Patient outcomes and stroke characteristics
Stroke characteristics and patient outcomes
Graphic representation of the correlation of core infarct volume and percentage brain infarction with the Pediatric Stroke Outcome Measure. TBV indicates total brain volume.
Infarct volume versus Pediatric Stroke Outcome Measure
A and B, CAV segmentation results from patients in good and poor outcome groups, respectively. C and D, Manual segmentation results from the same respective patients.
Discussion
Quantification of core infarct volume within the pediatric stroke population may provide assistance in clinical decision-making and prognostic information. However, challenges in manual segmentation currently limit its utility in an acute stroke setting because it can be prohibitively time-consuming. Additionally, while the present study did not find significant variability for manual measurements, prior work has suggested that manual determination of core infarct volume is a source of variance.27 In the present study, we compared the quantification of infarct volume and the percentage of infarcted brain parenchyma by using a semiautomated, computer-assisted approach with a traditional, manual approach and observed no significant difference in measured infarct volumes. CAV assessment showed high correlation between users, but the CAV was significantly faster compared with the manual approach, taking seconds to perform. The rapidity of computer-assisted quantification of infarct volume allows its real-time integration into a routine, clinical workload and its use in investigative research.
The development of quantitative neuroimaging biomarkers is needed to inform treatment planning, management, and prognostication in the setting of acute ischemic infarct in children as well as clinical trials, which need objective metrics for the assessment of clinical outcomes and cost effectiveness. Several prior studies have shown a correlation between final infarct volume and worse neurologic outcome. Zecavati et al11 observed that core infarct volumes in pediatric patients, as determined on DWI, that exceed 10% of brain parenchymal volume were associated with poor neurologic outcome at 30 days; however, the authors used the Glasgow Outcome Score, which, unlike the PSOM, has not been specifically validated in the pediatric population. The PSOM is based on a standardized, neurologic examination; is currently the best validated outcome measure; and has shown excellent interobserver correlation and validity and reproducibility when used retrospectively to analyze neurologic examinations or in prospective longitudinal research.20,21,28 In our study, we assessed the correlation between core infarct volume and clinical outcome by using the Pediatric Stroke Outcome Measure and observed that both infarct volume and the percentage of infarcted brain parenchyma correlated significantly with outcome. Specifically, cutoff values above 32 mL for core infarct volume and 3% for percentage of infarcted brain parenchymal volume were always associated with a bad outcome.
The CAV approach is robust; it is applicable to cases of solitary and multiple lesions and may represent an advance over qualitative vascular territory-based stroke scales. In addition, the software easily delineates total brain parenchymal volume excluding extra-axial CSF so that the percentage of infarcted brain parenchyma can be easily quantified. Beyond its utility in normalizing the infarct volume, CAV can be used independently for assessing total parenchymal or ventricular volume and may have additional applications in pediatric neuroradiology. Incorporation of CAV methods in standard clinical workflow has profound implications for the relevance of the radiologic consultation. In the context of pediatric stroke, CAV may identify children who would benefit from more aggressive therapy. CAV may quantify volumetric thresholds that could be used as end points for future clinical trials in pediatric stroke, and semiautomated methods may facilitate translation of these results from clinical trials into clinical practice.
When considering our results, several limitations should be kept in mind. First, we conducted a retrospective study, and our cohort of 23 patients is modest in size, which limits evaluation for confounders. Second, inclusion of neonates could be an additional confounder because longer follow-up may be needed because there may be a delay in the appearance of deficits after neonatal stroke, given that subtle sequelae may be difficult to delineate on neurologic examination in an incompletely myelinated, immature brain.20,28 However, the average age in our study was almost 5 years. Because our study was based at a tertiary referral center, the poor outcome category could have been over-represented. Children with associated neurologic disorders are included because stroke occurs with increased incidence in this group and gives the results wider applicability, and other investigators have validated the inclusion of children with neurologic disorders.28 Additionally, our study is based on calculation of infarct volume on DWI sequences in the acute setting within 72 hours of diagnosis. However, Ebinger et al12 have shown that subacute reduced diffusion volume in adults tightly correlated with final neurologic outcome assessed with the National Institutes of Health Stroke Scale, but most studies have shown stable DWI lesion evolution from 36 hours to 2 weeks.12,29
Conclusions
CAV quantification of core infarct volume in acute arterial ischemic infarct in pediatric patients provides a reproducible and significantly faster result comparable with manual techniques, which allows its easy integration into routine, clinical workflow. In our study, cutoff values above 32 mL for core infarct volume and 3% for percentage of infarcted brain parenchymal volume were associated with a poor outcome in all patients.
Footnotes
Disclosures: Christopher G. Filippi—RELATED: Grant: Radiological Society of North America Medical Student Seed Grant,* Comments: At the time of the study, coauthor Alexander El-Ali was a medical student; he is now a doctor, having graduated in May; UNRELATED: Consultancy: Regeneron Pharmaceuticals, Syntactx; Grants/Grants Pending: National Institutes of Health, Comments: 7/01/2012 to 4/30/2017; “Chronic Convection-Enhanced Delivery of Topotecan in Malignant Glioblastoma Multiforme”; Collaborator: C.G. Filippi (10% effort); Principal Investigator: Jeffrey Bruce; National Institutes of Health/National Cancer Institute; 1R01CA161404-01A; Other: advisor to resident and medical student as a research mentor,* Comments: Medical student and radiology residents have Radiological Society of North America grants for which I am scientific mentor, but this is unrelated to this work because it deals with T1 ρ MR imaging. *Money paid to the institution.
Alexander M. El-Ali, MD, is a recipient of Radiological Society of North America Medical Student Seed Grant 2013–2014.
Paper previously presented as an oral abstract at: Annual Meeting of the American Society of Neuroradiology, May 17–22, 2014; Montreal, Quebec, Canada.
REFERENCES
- Received June 4, 2014.
- Accepted after revision September 28, 2014.
- © 2015 by American Journal of Neuroradiology