Abstract
SUMMARY: Advances in neonatal neuroimaging have improved detection of preterm brain injury responsible for abnormal neuromotor and cognitive development. Increasingly sophisticated MR imaging setups allow scanning during early preterm life. In this review, we investigated how brain MR imaging in preterm infants should be timed to best predict long-term outcome. Given the strong evidence that structural brain abnormalities are related to long-term neurodevelopment, MR imaging should preferably be performed at term-equivalent age. Early MR imaging is promising because it can guide early intervention studies and is indispensable in research on preterm brain injury.
ABBREVIATIONS:
- DEHSI
- diffuse excessive high signal intensity
- FA
- fractional anisotropy
- PLIC
- posterior limb of the internal capsule
- PMA
- postmenstrual age
- PWML
- punctate white matter lesions
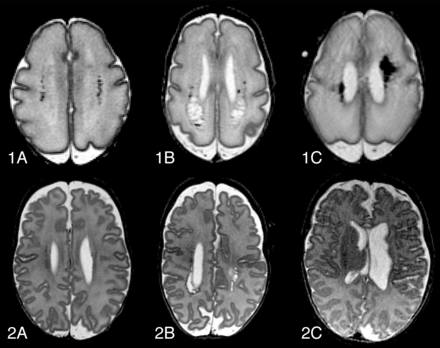
Preterm birth with subsequent brain injury is an increasing public health concern. Advances in neonatal intensive care have significantly improved survival rates among very-low-birth-weight infants, but survivors are still at considerable risk to develop cognitive, behavioral, neurosensory, and motor disabilities.1⇓⇓⇓–5 The most common preterm brain injury patterns are the following: WM injury; germinal matrix-intraventricular hemorrhage and its correlates; and posthemorrhagic ventricular dilation and periventricular hemorrhagic venous infarction (Fig 1). Cystic periventricular leukomalacia is seen less often now, and diffuse noncystic types of WM injury, including punctate WM lesions and diffuse excessive high signal intensity, are therefore most frequent6⇓⇓⇓–10 and the leading cause of disturbed brain growth, connectivity, and functionality.11⇓–13
Evolution of common types of preterm brain injury, at 30 weeks' postmenstrual age (1) and at term-equivalent age (2). Transversal T2-FSE images of punctate white matter lesions (A), periventricular leukomalacia (B), and periventricular hemorrhagic venous infarction (C). Note that images 2B and 2C are slightly oblique.
Although MR imaging is superior to cranial sonography in detecting diffuse WM injury,14⇓⇓–17 structural MR imaging studies fail to precisely predict outcome6,8,18 because conventional MR imaging is not sensitive enough to measure changes in microstructure.19 However, advanced MR imaging acquisition sequences and postprocessing techniques, such as DTI, volumetric MR imaging measurements, and proton MR spectroscopy (1H-MR spectroscopy), may be a solution. For example, DTI allows quantification of WM at a microstructural level by measuring the diffusion of water molecules in tissues.20,21
DTI studies have shown increasing fractional anisotropy and decreasing ADC during brain maturation, which is ascribed to the decreased water content and increased WM complexity due to myelination.20,22 Deviations from these developmental trends are considered diagnostic of perinatal WM injury.23⇓–25
WM injury in preterm infants has been related to significantly reduced brain volume,26,27 but brain growth in extremely preterm infants may also be disturbed in the absence of evident WM abnormalities. Volumes of brain regions and structures are correlated to perinatal complications and are inversely related to gestational age at birth.28,29 Smaller volumes are often associated with impaired neuropsychological function at a later age.29,30
Assessment of cortical folding during early brain development, with the use of postprocessing software,31 has provided insight into the underlying mechanisms of normal development, regional specialization, and functional lateralization.32,33 Anomalous cortical folding, demonstrated in preterm infants, has been proposed as an early biomarker of neurocognitive impairment.34,35
Metabolic integrity of tissues can be measured in vivo with 1H-MR spectroscopy. The NAA/Cho ratio is of special interest in neonatal neuroimaging because the ratio increases during brain maturation as an effect of synthesis by proliferating oligodendrocyte progenitor cells.36
Early MR imaging provides early biomarkers of preterm brain injury and enables early parental counseling. However, systematic use of such MR imaging has its limitations due to hemodynamic, respiratory, and thermodynamic instability seen in most preterm infants.37 Moreover, technical aspects like smaller heads result in lower SNR.38 As in most studies obtained at term-equivalent age,18,30 less is known about the value of scanning at a lower postmenstrual age. Furthermore, brain injury can also occur in the late preterm period. MR imaging at term has the disadvantage of parents and caregivers not being fully informed until their child reaches term age. Furthermore, logistic issues may emerge in centers where infants are transferred to other hospitals once certain criteria are met.
Because there seems to be no consensus on the optimal timing of MR imaging, we reviewed the literature on the prediction of neurodevelopmental outcome with the use of brain MR imaging performed at either early preterm or term age.
Materials and Methods
The Embase, MEDLINE OvidSP, Cochrane, and PubMed databases were systematically searched for relevant articles published between 1979 and November 2012. The strategy included synonyms and combinations of the following keywords: “prematurity, ” “neuroimaging, ” “brain, ” and “MR imaging ” (full research strategy is available on-line). The search was limited to human research that involved original patient data, and only articles written in English were included.
Studies were eligible under the following conditions: 1) they included preterm infants born at <32 week' gestation, 2) MR imaging was performed in the neonatal period, and 3) neurodevelopmental outcome was linked to MR imaging findings. To avoid large variations in MR imaging determinants, we only included structural MR imaging studies if they evaluated the findings according to a reproducible classification.
The initial search resulted in 2104 citations. Two reviewers (A.P., J.D.) screened all abstracts of these citations for relevance and reached a consensus after discussion in case of disagreement. Sixty-two articles were incorporated in this review. In the “Results ” section, we present findings according to type of MR imaging technique: conventional structural MR imaging, such as T1- and T2-weighted scans, DTI, volumetric MR imaging, and proton MR spectroscopy. Further classification was based on the timing of MR imaging: serial, before 35 weeks', or after 35 weeks' PMA.
Results
Conventional Structural MR Imaging
Serial MR Imaging.
Three serial neuroimaging studies correlated injury to outcome (Table 1). One was a prospective consecutive MR imaging study by Dyet et al,8 regarding 327 MR imaging scans of 119 preterm infants. Only major destructive cerebral and cerebellar lesions seen at the initial scan within 2 days after birth were related to poorer neurodevelopmental outcome. DEHSI and posthemorrhagic ventricular dilation at term-equivalent age were significantly related to adverse outcome. Isolated hemorrhage or PWML did not seem to predict adverse neurodevelopmental outcome. The second, by Miller et al,39 demonstrated that moderately severe abnormalities, such as WM injury, ventriculomegaly, and intraventricular hemorrhage on early scans were associated with adverse neurodevelopmental outcome as strongly (or even more strongly) as abnormalities on the term-equivalent scans: The relative risk was 5.6 and 5.3, respectively. The third, a large serial MR imaging study by Tam et al,40 demonstrated that not only large but also small cerebellar hemorrhages, not detected on cranial sonography, were associated with abnormal neurologic examination at 3–6 years of age. The presence of these small cerebellar hemorrhages was associated with a 5.0 odds ratio of abnormal neurologic examination findings at a mean age of 4.8 years.
Details of included serial MRI studies
MR Imaging at ≤35 Weeks' PMA.
The presence of cystic periventricular leukomalacia and cerebellar hemorrhage at 35 weeks' gestation was significantly correlated to abnormal neurologic examination findings at 30 months in a retrospective neuroimaging study by Cornette et al.41 Isolated PWML was not correlated to abnormal neurodevelopmental outcome at 30 months of age (Table 2).
Details of included MRI studies, scanned at ≤35 weeks' postmenstrual age
MR Imaging at >35 Weeks' PMA.
Twenty-six studies correlated brain injury or conventional MR imaging at 35 weeks' PMA with outcome (On-line Table).
The impact of overt WM lesions at term on neurodevelopment has been extensively investigated. The severity of WM abnormalities is often assessed according to a comprehensive scoring system15 and is assumed to be directly associated with the incidence of neuromotor impairment until 5 years of age9,10,15⇓–17,42⇓⇓⇓⇓⇓⇓–49 and to be inversely correlated to the Bayley scales50 until 30 months15,16,43,51⇓⇓–54 and cognitive performance until 9 years of age.55⇓⇓⇓⇓–60 The presence of WM injury has an odds ratio of 8.3 for low full-scale intelligence quotient (IQ < 70).59 Moderate-to-severe WM abnormalities highly predict severe motor delay; odds ratios up to 10.0 and positive predictive values up to 100% have been demonstrated.15,42,44,45,52,59
The association between subtle diffuse WM injury and neurodevelopmental outcome is not clear.61 Some research groups demonstrated a significant association between PWML and impaired neurodevelopmental outcome,10,46,62 whereas others suggested the contrary, provided that no other major lesions were observed.8,52 DEHSI was associated with adverse outcome in a large serial imaging study by Dyet et al,8 but others could not confirm this finding.10,42,51,59,62,63 The lack of clarity is thought to be due to the absence of objective definitions for these patterns of brain injury24,48,64 and raises the importance of objective assessment of diffuse WM injury.
Extensive intraventricular hemorrhage and venous infarctions, according to Papile et al,65 are associated with neurodevelopmental impairment.16,17,48,53 Posthemorrhagic ventricular dilation is associated with neurologic impairment until 6 years of age.66 In a study by De Vries et al,67 asymmetric myelination of the PLIC at term age in preterm infants with venous infarction seemed to be an early predictor of future hemiplegia.
Although commonly described in cranial sonographic studies,68 caudothalamic cysts were not related to cognitive and neuropsychological impairment in a MR imaging study by Lind et al.69
The impact of gray matter abnormalities remains unclear. They were significantly associated with abnormal neurobehavioral outcome at term in a study by Brown et al47 and with decreased Bayley scales at 2 years in a study by Woodward et al,15 but others9,59 found no significant relationship between injury to the cerebral gray matter and neuromotor function at term9 or cognitive outcome at 9 years of age.59
Diffusion Tensor Imaging
Serial MR Imaging.
Two serial DTI studies found a significant correlation with cognitive and neurosensory outcome (Table 1). Drobyshevsky et al70 demonstrated that the Bayley performance index at 24 months was correlated with FA of the PLIC at 30 weeks (r = 0.55) and faster increase of FA per week in the internal capsule (r = −0.63) and occipital WM (r = −0.59). Increased FA values in the optic radiation at 33 and 37 weeks were associated with increased visual-evoked-response amplitudes at 10.5 months (r = 0.7).71 However, this may not necessarily mean that eventually visual function is better.
MR Imaging at ≤35 Weeks' PMA.
None of the included studies related early DTI measurements to long-term outcome.
MR Imaging at >35 Weeks' PMA.
In a tract-based spatial statistics study by van Kooij et al,72 FA values of the corpus callosum were correlated to cognitive scores, gross motor scores were correlated with radial diffusion of the corpus callosum and internal and external capsules, and fine-motor scores were correlated to FA throughout the WM. Other DTI studies have demonstrated similar correlations: DTI parameters of the corpus callosum, PLIC, right orbital frontal cortex, and centrum semiovale were correlated to cognitive performance (On-line Table).73⇓⇓–76 In other studies, DTI measurements of the corpus callosum, PLIC, and corona radiata were correlated to motor function.74,77⇓–79 Furthermore, FA values of the optic radiation were directly correlated to visual assessment scores at term-equivalent age.80
Volumetric MR Imaging
Serial MR Imaging.
Three serial volumetric MR imaging studies demonstrated that early structural abnormalities are predictors of neurobehavioral outcome (Table 1). Dubois et al81 concluded that at term-corrected age, neurobehavioral development was significantly associated with quantitative surrogates of cortical folding. Kapellou et al82 found that the ratio between cortical surface area and cerebral volume was directly related to neurodevelopment at 24 months. The same group showed that growth of the cortical surface area was also significantly related to intelligence at 6 years: A faster growth of 0.032% per week resulted in an increase of 1 IQ point.83
MR Imaging at ≤35 Weeks' PMA.
Badr et al84 found that WM volume on MR imaging at a mean PMA of 31 weeks was significantly correlated to the Bayley Psychomotor Development Index (r = 0.29) and Mental Development Index (r = 0.31) at 18 months (Table 2).
MR Imaging at >35 Weeks' PMA.
Volumetric MR imaging studies in preterm infants with neurodevelopmental impairment have demonstrated significantly smaller total brain volume54,66,85 and volume of several cerebral structures or regions, including the cerebellum,66,86⇓⇓–89 total WM,90 total28,91 and deep66,76 gray matter, occipital lobes,92 hippocampus,93,94 and brain stem,95 as well as significantly larger ventricles (On-line Table).28,96 These findings were irrespective of the presence of overt brain injury. Simple linear metric assessment, such as biparietal and cerebellar diameter, on MR imaging also significantly correlated with neurocognitive function.97,98 Impaired social-emotional development at 5 years was associated with decreased hippocampal volume in girls and decreased frontal lobe growth in boys.75
Proton MR Spectroscopy
MR Imaging at >35 Weeks' PMA.
1H-MR spectroscopy is an accurate quantitative biomarker for the prediction of neurodevelopmental outcome after hypoxic-ischemic encephalopathy in term infants (On-line Table).99 It is not clear whether this holds true for preterm infants. The cerebellar NAA/Cho ratio at term is suggested to correlate with cognitive outcome at 24 months.89 However, Gadin et al91 found no correlation between MR spectroscopy of the periventricular WM and motor development at 6 months.
Discussions
This systematic review included 8 serial MR imaging studies, 2 MR imaging studies performed at ≤35 weeks, and 52 MR imaging studies performed at >35 weeks. The results of these studies made clear that the extent of structural abnormalities, microstructural deviations, and global reductions in brain volumes, both at preterm and term age, is directly related to the level of neuromotor and neurocognitive performance in childhood. Involvement of WM in preterm brain injury seems paramount. Accurate assessment of WM integrity, therefore, may help predict long-term outcome in preterm infants and is one of the challenging goals in the field of neonatal neurology.
These studies do not provide clear evidence on the optimal timing of MR imaging. Although an increasing number of neuroimaging studies used early MR imaging to show that brain abnormalities are often present during early preterm life,22,100,101 only 2 of the studies linked these findings to outcome. Dyet et al8 demonstrated that MR imaging within the first 2 days after birth was of limited additional value for predicting outcome. On the other hand, Miller et al39 reported that early MR imaging findings at 32 weeks' gestation were as reliable for predicting neurodevelopment as MR imaging findings at term age. This finding suggests that predictive MR imaging may be performed well before term-equivalent age, provided it is after the first week of life.
Neonatal care would benefit from identifying brain injury early in preterm life, in terms of effective and timely parental counseling, tailored rehabilitation strategies, and better understanding of neuropathology. Currently, we have no efficacious therapy for preterm brain injury, but trials on possible neuroprotective agents, such as erythropoietin, melatonin, stem cell therapy, and magnesium sulfate are being conducted or planned for the near future.102,103 Early MR imaging could provide early biomarkers that trials could target.
Image acquisition, processing, and interpretation are not as straightforward as with conventional MR imaging, though sophisticated techniques such as DTI allow objective and quantifiable assessment of cerebral tissue. Because measurement accuracy depends on various aspects, including scanner type, hardware setup, acquisition settings, and clinical characteristics, reproducibility of the same measurements in different imaging centers is low. Furthermore, the availability of normal ADC and FA values of specific WM structures is limited. In addition, DTI is especially sensitive to image artifacts and corruption.104 Reliable conclusions can therefore only be drawn if quality assessment before postprocessing provided satisfactory data quality. In the included studies, quality assessment was often not performed.
MR imaging is expensive and time-consuming and requires great experience and dedication to ensure patient safety37 as well as good quality data and interpretation.105 These limitations should be especially taken into account with regard to the individual clinical care for patients with normal cranial sonography findings. This technique can reliably predict some aspects of the outcome of preterm infants and allows serial neuroimaging in a fast, convenient, and less-expensive manner.106,107 Moreover, advanced applications, such as color Doppler sonography, also allow objective and quantitative brain assessment.
Several limitations of this systematic review need to be addressed. First, heterogeneity of the study populations was due to variation in age at MR imaging, acquisition settings, postprocessing methods for MR imaging evaluation and other technical aspects of MR imaging scanners, different ages at outcome measurement, and different measures of outcome. Second, follow-up periods were relatively short. Third, because the search was restricted to articles in the English language, possible relevant studies might not have been included.
Conclusions
MR imaging remains an outstanding method to predict long-term neurodevelopmental outcome, and cerebral MR imaging should be part of standard clinical care for preterm infants. Early MR imaging allows timely parental counseling, targeting of rehabilitation strategies, and availability of early biomarkers. However, the individual prognostic information provided by early scanning remains inferior to that provided by term scanning. As long as the correlation of brain injury from early MR imaging with outcome is not clear, we would argue that standard MR imaging should preferably be performed at term-equivalent age. On the other hand, early MR imaging yields important information about the pathogenesis of preterm brain injury and therefore is indispensable in research on preterm brain injury.
Acknowledgments
We thank Wichor Bramer from the Erasmus Medical Center medical library for his help in devising the search strategy.
Indicates open access to non-subscribers at www.ajnr.org
REFERENCES
- 1.↵
- 2.↵
- 3.↵
- 4.↵
- 5.↵
- 6.↵
- 7.↵
- 8.↵
- 9.↵
- 10.↵
- 11.↵
- 12.↵
- 13.↵
- 14.↵
- 15.↵
- 16.↵
- 17.↵
- 18.↵
- 19.↵
- 20.↵
- 21.↵
- 22.↵
- 23.↵
- 24.↵
- 25.↵
- 26.↵
- 27.↵
- 28.↵
- 29.↵
- 30.↵
- 31.↵
- 32.↵
- 33.↵
- 34.↵
- 35.↵
- 36.↵
- 37.↵
- 38.↵
- 39.↵
- 40.↵
- 41.↵
- 42.↵
- 43.↵
- 44.↵
- 45.↵
- 46.↵
- 47.↵
- 48.↵
- 49.↵
- 50.↵
- 51.↵
- 52.↵
- 53.↵
- 54.↵
- 55.↵
- 56.↵
- 57.↵
- 58.↵
- 59.↵
- 60.↵
- 61.↵
- 62.↵
- 63.↵
- 64.↵
- 65.↵
- 66.↵
- 67.↵
- 68.↵
- 69.↵
- 70.↵
- 71.↵
- 72.↵
- 73.↵
- 74.↵
- 75.↵
- 76.↵
- 77.↵
- 78.↵
- 79.↵
- 80.↵
- 81.↵
- 82.↵
- 83.↵
- 84.↵
- 85.↵
- 86.↵
- 87.↵
- 88.↵
- 89.↵
- 90.↵
- 91.↵
- 92.↵
- 93.↵
- 94.↵
- 95.↵
- 96.↵
- 97.↵
- 98.↵
- 99.↵
- 100.↵
- 101.↵
- 102.↵
- 103.↵
- 104.↵
- 105.↵
- 106.↵
- 107.↵
- © 2014 by American Journal of Neuroradiology