Abstract
BACKGROUND AND PURPOSE: Acute unilateral optic neuritis is associated with a thickening of the retrobulbar portion of the optic nerve as revealed by transorbital sonography, but no comparison has been made between nerve sheath diameter and optic nerve diameter in patients with acute optic neuritis versus healthy controls. We evaluated optic nerve sheath diameter and optic nerve diameter in patients with acute optic neuritis and healthy controls and compared optic nerve sheath diameter and optic nerve diameter with visual-evoked potentials in patients.
MATERIALS AND METHODS: A case-control study was performed in 2 centers. Twenty-one consecutive patients with onset of visual loss during the prior 10 days and established acute noncompressive unilateral optic neuritis were compared with 21 healthy controls, matched for sex and age (±5 years). Two experienced vascular sonographers performed the study by using B-mode transorbital sonography. Visual-evoked potentials were performed on the same day as the transorbital sonography and were evaluated by an expert neurophysiologist. Sonographers and the neurophysiologist were blinded to the status of the patient or control and to clinical information, including the side of the affected eye.
RESULTS: The median optic nerve sheath diameter was thicker on the affected side (6.3 mm; interquartile range, 5.9–7.2 mm) compared with the nonaffected side (5.5 mm; interquartile range, 5.1–6.2 mm; P < .0001) and controls (5.2 mm; interquartile range, 4.8–5.5 mm; P < .0001). The median optic nerve diameter was 3.0 mm (range, 2.8–3.1 mm) on the affected side and 2.9 mm (range, 2.8–3.1 mm) on the nonaffected side (P = not significant.). Both sides were thicker than those in controls (2.7 mm; interquartile range, 2.5–2.8 mm; P = .001 and .009). No correlation was found between optic nerve sheath diameter and optic nerve diameter and amplitude and latency of visual-evoked potentials in patients with optic neuritis.
CONCLUSIONS: Transorbital sonography is a promising tool to support the clinical diagnosis of acute optic neuritis. Further studies are needed to define its specific role in the diagnosis and follow-up of optic neuritis.
ABBREVIATIONS:
- ON
- optic neuritis
- OND
- optic nerve diameter
- ONSD
- optic nerve sheath diameter
- TOS
- transorbital sonography
- VEP
- visual-evoked potentials
Optic neuritis (ON) is an acute inflammation of the optic nerve that may cause a complete or partial loss of vision. The classic triad for its clinical diagnosis is visual loss, periocular pain, and dyschromatopsia.1 ON typically occurs in young adults with an approximately 3:1 female-male ratio. ON is mostly idiopathic but may be associated with autoimmune disorders and infectious, inflammatory, and demyelinating diseases, especially multiple sclerosis. It is diagnosed through a complete ophthalmologic and neurologic evaluation and a prolonged latency of the visual-evoked potentials (VEP). MR imaging with gadolinium is valuable for differential diagnosis.2
Previous studies have shown that transorbital sonography (TOS) reliably investigates the optic nerve owing to its low reflectivity and the high reflectivity of the perineural sheath and orbital fat.3 TOS has been recognized as an accurate, noninvasive method to identify papilledema.4,5 Some TOS studies have shown that the optic nerve may be enlarged in acute ON,6⇓⇓⇓⇓–11 though the validity of this technique for the diagnosis of ON has not yet been recognized.12 No sonographic data are available to distinguish enlargement of the nerve itself from that of its sheath, though an enlargement of the sheath in acute ON has been described with MR imaging.13
Previous studies did demonstrate good accuracy and reliability of sonographic quantification of optic nerve sheath diameter (ONSD).14,15
The primary aim of this study was to use TOS to assess enlargement of the optic nerve and its sheath in acute ON by measuring optic nerve diameter (OND) and ONSD. The second aim was to identify whether OND and ONSD values were associated with any VEP abnormalities in cases of acute ON.
Materials and Methods
Patients
All consecutive patients presenting to the Neurology Outpatient Clinics of the Novara and Merano Hospitals between December 2012 and October 2013 with a diagnosis of the first acute episode of demyelinating unilateral ON were invited to enter the study. The study was approved by the local ethics committees and was performed in accordance with the Declaration of Helsinki. Written informed consent was obtained from all persons before entering the study.
All patients underwent neurologic and ophthalmologic examinations, including visual acuity assessment, direct ophthalmoscopy, and laboratory examinations, including vasculitis screening and antineuromyelitis optica antibodies. MR imaging was performed to exclude other causes of ON or compressive lesions. Inclusion criteria were 18–50 years of age, visual loss with onset <10 days before the visit, and diagnosis of ON according to the Optic Neuritis Study Group.16 Exclusion criteria were bilateral or recurrent ON, suspected ischemic optic neuropathy, recent steroid treatment, and recent lumbar puncture.
Controls were 21 healthy volunteers, matched to patients by sex and age (±5 years) and selected among students, university personnel, relatives of patients admitted to the hospital, and their friends. Controls were examined to exclude a diagnosis of MS, ON, or other neurologic diseases; those with a relative affected by MS were excluded.
Patients and controls with a history of any major systemic diseases, including cardiovascular disease, arterial hypertension, hyperlipidemia, or diabetes, and pregnant or breast-feeding women were excluded. The interval from the onset of symptoms was calculated from the first day the patient experienced visual loss or retro-orbital pain to the date of TOS.
Procedure
TOS was performed in patients and age- and sex-matched controls by 2 expert sonographers (P.L. and L.C.). VEP were performed only in patients, to avoid discomfort due to needle electrode placement in healthy controls, and were evaluated by an expert neurophysiologist (G.S.). TOS and VEP were always performed on the same day and before the onset of steroid treatment. Operators were unaware of the side involved (sonographers, VEP technicians, neurophysiologist) and of the condition of patients or controls (sonographers). To ensure blinding, we asked patients and healthy controls not to reveal their affected side (or their status) during examinations, and they were always placed on the tilt table before the arrival of the sonographer.
Transorbital Sonography
TOS was performed in B-mode by using a Vivid 7 sonography system with a 7- to 11-MHz linear array transducer (GE Healthcare, Milwaukee, Wisconsin) and an Aplio XG equipped with a 4- to 11-MHz 5 S1 Linear Probe (Toshiba Medical Systems, Nasu, Japan). A procedure described previously in the literature was used.14,15 Subjects were examined in the supine position with the upper part of the body and the head elevated to 20°–30° to avoid any pressure on the eye and were asked to keep their eyes in a midposition and to suppress eye movements.
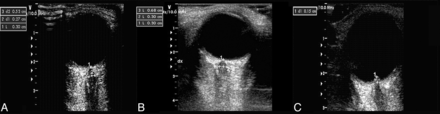
For safety reasons due to possible biomechanical side effects, the mechanical index was reduced to 0.2. The probe was placed on the temporal part of the closed upper eyelid by using a thick layer of sonography gel. The anterior part of the optic nerve was depicted in an axial plane showing the papilla and the optic nerve in its longitudinal course. ONSD and OND were assessed 3 mm behind the papilla. To measure the ONSD, we quantified the distance between the external borders of the hyperechogenic area surrounding the optic nerve. We measured the OND, marking the internal borders of this formation (Fig 1). To minimize intraobserver variability, we examined each bulb 3 times and calculated the means. Sonography was also used to evaluate the presence of papilledema.4,5 The presence of papilledema, evaluated by assessing optic disc elevation, was measured between the fundus and the dome of the papilla (Fig 1). Agreement between the 2 sonographers was preliminarily assessed for ONSD and OND by using the same device in 9 healthy subjects (see “Statistical Evaluation”).
Transorbital sonography in optic neuritis. A–C, Sonographic examinations of the eye are performed in B-mode imaging in a control subject. A, Optic nerve sheath diameter in the control eye is 3 mm behind the papilla (1) (dotted arrow) in an axial plane showing the optic nerve (2) in its longitudinal course. The dotted arrow (3) denotes the ONSD. B, B-scan shows an optic neuritis increase of ONSD (left, 6.8 mm). C, In patients with optic neuritis with disc swelling, optic disc elevation is gauged between the fundus and the dome of the papilla.
Pattern-Reversal Visual-Evoked Potentials
Standard pattern-reversal VEP were performed according to recent guidelines17 with 4-channel equipment (Medtronic Keypoint, Denmark). The visual stimulus was a black-and-white pattern checkerboard (24 × 32) with 90% contrast, generated on an LED monitor (17-inch, high-resolution display; Acer, New Taipei City, Taiwan), with stimulus frequency, 2 Hz; observation distance, 100 cm. Patients were preadapted to the room lighting, and all recordings were performed under dim room lights. Patients were instructed to fix on the red point in the center of the screen with their best refractive correction during the monocular stimulation. The recording electrode was placed on Oz, the reference electrode was placed on Cz, and the ground electrode, on Fz according to the International 10–20 System. The impedance was kept below 3 kΩ on all electrodes. Signals were amplified and filtered with a bandpass filter from 0.2 to 3 kHz. One hundred responses were averaged for each side. Latencies of the N75, P100, and N145 waves and amplitude of the N75-P100 complex were analyzed.
Statistical Evaluation
If one assumed a median ONSD in controls of 5.0 mm, the sample needed to detect a mean difference of 1 mm was 10 patients and 10 controls with an α error of .05 and a β error of 0.20. Continuous variables were described by their median with the interquartile range. Comparisons between groups were assessed by using parametric (Student t test) and nonparametric methods (Wilcoxon test, Fisher exact test, and Spearman correlation) when appropriate (deviation from normal distribution according to the Shapiro-Wilk test). In view of the large number of comparisons, the Bonferroni correction for statistical significance was used.
Interobserver agreement between the ONSD and OND evaluations made by the 2 sonographers was preliminarily assessed with intraclass correlation coefficients.
Results
We analyzed 21 patients with a first episode of ON, 17 women and 4 men; a visual loss >3/10 was present in 18 (85.7%). The right eye was affected in 16, and the left, in 5. Eleven patients had prior MS relapses (ON-MS), though never involving ON, and 10 had isolated ON. We enrolled 21 healthy controls. Mean age and body mass index were not different between patients and controls (Table). The Table reports clinical features and ONSD and OND values of patients and controls. Interobserver agreement was high for both ONSD (intraclass correlation coefficient, 0.98; 95% CI, 0.93–1.00) and OND (intraclass correlation coefficient, 0.98; 95% CI, 0.92–1.00). The main finding of our study was the statistically significant thickening of the ONSD on the affected side (intraclass correlation coefficient, 6.3 mm; 95% CI, 5.9–7.2 mm) compared with the nonaffected side (intraclass correlation coefficient, 5.5 mm; 95% CI, 5.1–6.2 mm; P < .0001) between patients and controls (Table). The median OND in the affected side (3.0 mm; range, 2.8–3.1 mm) was similar to that in the nonaffected side (2.9 mm; range, 2.8–3.1 mm) (P = not significant). Both sides were thicker than those in controls (median, 2.7 mm; range, 2.5–2.8 mm; P = .001 and .009). The OND and ONSD were strictly correlated both in healthy controls (r = 0.63, P = .002) and the nonaffected eye (r = 0.45, P = .039) but not in the affected eye (r = −0.15, P = .51).
Clinical and sonographic features of patients with optic neuritis and healthy control subjects
Among controls, the ONSD or OND was similar in men and women. The median ONSD was 4.8 mm (interquartile range = 4.8–5.3 mm) in men and 5.3 mm (interquartile range = 4.8–5.6 mm) in women (P = .16); the median OND was 2.5 mm (interquartile range = 2.3–2.8 mm) in men and 2.7 mm (interquartile range = 2.5–2.9 mm) in women (P = .17). Neither ONSD (r = 0.37) nor OND (r = 0.13) was correlated with age (P = not significant).
We did not find any significant difference between the ONSD and OND in patients with ON-MS and in those with isolated ON (Table).
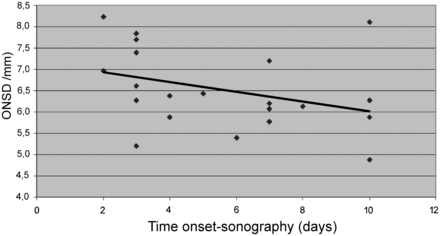
An almost significant inverse correlation between the delay of TOS examination and ONSD diameter in the affected eye was found, suggesting that the earlier the examination, the larger was the diameter (r = −0.42, P = .06) (Fig 2). No correlation was found between delay and OND (r = −0.11, P = .62).
Optic nerve sheath diameter and delay from symptom onset. Correlation between the delay of the transorbital sonography examination (days from symptom onset) and optic nerve sheath diameter in the affected eye (r = −0.42, P = .06).
TOS revealed papilledema in 9 patients (43%), 8 on the right side, and in none of the controls. There was no statistical difference between patients with and without this elevation in terms of age, body mass index, ONSD, OND, VEP latency, and VEP amplitude (data not shown).
No correlation (P = not significant) was found between the ONSD and either latency or amplitude of VEP both in the affected (latency, r = −0.01; amplitude, r = 0.07) and the fellow eye (latency, r = 0.05; amplitude, r = 0.09). No correlation (P = not significant) was found between the OND and either latency or amplitude of VEP both in the affected (r = 0.22; −0.16, respectively) and the fellow eye (r = 0.23; 0.31, respectively).
Discussion
Our study is the first to report transorbital sonographic measurement of both the ONSD and OND in patients with acute ON. Patients with ON had significantly increased ONSD values in the affected eye compared with the other eye and with values in age-matched controls. The thickening of the perineural space surrounding the optic nerve is probably related to inflammation of the optic nerve, resulting in an increase of the perineural subarachnoid fluid or edema caused by an impairment of axoplasmic flow, depending on the acute demyelinating plaque. Most interesting, it has been reported that narrowed optic canals, occurring for instance in osteopetrosis, may lead to compressive optic neuropathy.18,19 It is, therefore, possible that within the anatomic variability among different subjects, a tendency toward reduced diameter of the optic canals may lead to a further increase in perineural subarachnoid fluid and edema, with subsequent worsening of visual dysfunction. Our results, therefore, suggest that ONSD has a high sensitivity for the diagnosis of acute ON. Our study was not designed to assess the specificity and predictive value of ONSD, which would require a larger sample size and controls with different diseases and different settings. Most interesting, although our patient population is not different from that in previous studies,6⇓⇓⇓⇓–11 the mean ONSD was slightly higher and corresponded to the ONSD values of patients with either chronically4 (6.4 mm) or acutely elevated intracranial pressure (6.2 mm).20 We believe that this discrepancy could be explained by a different resolution of the sonography system, different probes, or by variability in the evaluation of sonographic anatomy and time of insonation. We also found that the sooner TOS is performed after symptom onset, the higher is the probability of detecting an increased ONSD. This observation supports the planning of further studies aiming to evaluate TOS for monitoring the evolution of inflammation.
The measurement of optic disc elevation is useful for detecting the presence of disc swelling (papilledema). Papilledema results from transmission of increased intracranial pressure to the subarachnoid space of the optic nerve, with compression of the nerve, stasis of axonal transport, and subsequent swelling of the optic nerve axons, particularly in patients with a narrow optic canal.21 In our study, we found a percentage of papilledema (45%) higher than that in previous studies (ranging from 6% to 37%).6⇓–8,10
Although the OND in the affected eye was not thicker than that in the contralateral eye, both were slightly thicker than that in matched controls. We have no clear explanation for this finding. Such a difference may be due to chance alone, or it may suggest a subclinical involvement of the fellow nerve. We did not find any correlation between TOS and VEP measures, though VEP were performed only in patients. VEP specifically evaluate the conduction time of the visual pathways, including the optic nerve tract: A slow conduction time (ie, prolonged latency of P100) is primarily due to a demyelinating process affecting the optic nerve. TOS instead evaluates the optic nerve and a different structure, its perineural space. Thus, these 2 techniques provide different, though complementary, information on the pathophysiology of ON.
This study is limited by the relatively small number of patients, the impossibility of obtaining a complete blinding of the clinical status of the patients, and the VEP performed only in patients. This study has several strengths, however, because we always performed sonographic assessments before starting steroid therapy and close to the onset of symptoms, and examiners were blinded to both clinical diagnosis and the side of ON. Furthermore, preliminary assessment of interobserver agreement between the ONSD and OND evaluations made by the 2 sonographers was high and similar to that found in a previous study by Bäuerle et al14 (r = 0.92–0.97). These findings indicate that sonographic ONSD quantification can be performed with good accuracy and reliability.
Conclusions
ONSD measured by TOS is a noninvasive, inexpensive, and easy procedure, which may represent a promising tool to support and confirm the clinical diagnosis of acute ON, even if it can only examine the anterior portion of the optic nerve. Further investigations with larger sample sizes and longitudinal studies are required to confirm our results.
Footnotes
Disclosures: Francesco Brigo—UNRELATED: Payment for Lectures (including service on Speakers Bureaus): UCB Pharma. Cristoforo Comi—UNRELATED: Grants/Grants Pending: Italian Ministry of Health,* Guillain-Barré Syndrome - Chronic Inflammatory Demyelinating Polyneuropathy Foundation International,* Comments: grants for research in neuroimmunology (€300,000), Travel/Accommodations/Meeting Expenses Unrelated to Activities Listed: Biogen, UCB Pharma, Novartis, AbbVie, Comments: meeting expenses (€5000). Maurizio A. Leone—UNRELATED: Board Membership: European Journal of Neurology, Comments: Associate Editor, Grants/Grants Pending: Fondazion Comunità del Novarese,* Comments: grant for homecare project in multiple sclerosis. *Money paid to the institution.
References
- Received March 24, 2014.
- Accepted after revision May 22, 2014.
- © 2014 by American Journal of Neuroradiology