Abstract
SUMMARY: The TMAs are a group of microvascular occlusive disorders characterized by thrombocytopenia and intravascular hemolysis. Literature review reveals a spectrum of neuroimaging findings, including a single case report of multifocal hemorrhagic infarctions. We present a series of 12 patients with TMA demonstrating a similar pattern of multifocal cortical and subcortical hemorrhagic infarctions.
Abbreviations
- ADC
- apparent diffusion coefficient
- AML
- acute myelogenous leukemia
- CLL
- chronic lymphocytic leukemia
- CNS
- central nervous system
- CTV
- CT venography
- DIC
- disseminated intravascular coagulation
- DWI
- diffusion-weighted imaging
- GRE
- gradient recalled-echo
- HIV
- human immunodeficiency virus
- HUS
- hemolytic uremic syndrome
- mHTN
- malignant hypertension
- MRI
- MR imaging
- MRV
- MR venography
- PRES
- posterior reversible encephalopathy syndrome
- SWI
- susceptibility-weighted imaging
- TMA
- thrombotic microangiopathy
- TTP
- thrombotic thrombocytopenic purpura
- vWF
- von Willebrand factor
TMA was defined by Symmers in 19521 as a lesion of arteriole and capillary wall thickening with swelling or detachment of endothelial cells from the basement membrane, accumulation of material in the subendothelial space, and lodging of fibrin-platelet thrombi at the arteriocapillary junction.2 The mechanical injury to erythrocytes results in intravascular hemolysis and schistocytosis. The intraluminal platelet thrombosis results in partial or complete obstruction of the vessel lumina.2
There is a spectrum of microvascular occlusive disorders in which endothelial injury incites platelet aggregation with thrombus formation and vascular occlusion, which includes TTP, HUS, DIC, autoimmune diseases, drug/toxin exposure, and mHTN. These clinically manifest as end-organ ischemia/infarction and/or hemorrhage.3,4 In TTP, brain lesions prevail, while renal lesions predominate in HUS.2 The incidence of TMA varies with the underlying diagnosis. Miller et al5 reported an age-sex standardized incidence of TTP and HUS of 6.5 per million per year in the United States. They found a greater incidence in females.5 This is generalized to systemic disease and not exclusively CNS involvement. Most cases of HUS occur before 20 years of age.5
Literature review reveals a spectrum of TMA neuroimaging findings including parenchymal hematoma, PRES, ischemic infarction, and venous thrombosis, with 1 case report of multifocal hemorrhagic infarctions.3,6,7 It is the latter imaging manifestation on which our case series focuses. We present a series of 12 patients with TMA sharing a similar neuroimaging pattern of multifocal cortical and subcortical hemorrhagic infarctions (Fig 1).
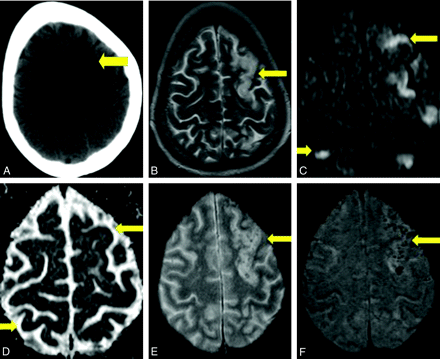
Case 6. A, Axial noncontrast CT scan demonstrates nonspecific peripheral hypoattenuation (arrow). B, Axial T2 MR image demonstrates cortical and subcortical hyperintensity (arrow) without appreciable hemorrhage. C, B = 1000 DWI MR image reveals multifocal cortical and subcortical hyperintensities (arrows). D, The corresponding apparent diffusion coefficient map demonstrates hypointensities in a similar distribution (arrows), consistent with infarction. E and F, GRE MR image reveals subtle blooming from petechial hemorrhage (E, arrow), better demonstrated on the SWI MR image (F, arrow).
Case Series
The cohort of 12 patients was obtained by 2 means. The first group of 7 patients was acquired via an institutional review board−approved retrospective search of the electronic medical records at our institution from 1997 through 2008 for all patients diagnosed with TTP, DIC, and/or mHTN. The patient list was cross-referenced with our imaging data base. Three neuroradiologists reviewed all neuroimaging, and the findings were tabulated. The second group of 5 patients was obtained from a review of our teaching file data base. Archived cases demonstrating multifocal cortical and subcortical hemorrhagic infarctions were included (Fig 2). An in-depth chart review was performed on all 12 patients to confirm the diagnosis of TMA.
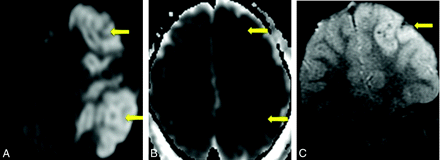
Case 12. A, B = 1000 DWI MR image reveals multifocal cortical and subcortical hyperintensities (arrows). B, The corresponding apparent diffusion coefficient map demonstrates hypointensities in a similar distribution (arrows), consistent with infarction. C, GRE MR image reveals petechial hemorrhage (arrow) in a similar distribution.
The electronic medical record search yielded 623 patients with TMA, 249 (40%) with neuroimaging during admission. Of the 249 patients, 7 (3%) demonstrated the imaging pattern of multifocal cortical and subcortical hemorrhagic infarctions. Review of the teaching file data base yielded an additional 5 cases with the same imaging pattern. Patient demographics, imaging modalities, and clinical information are summarized in the Table.
Patient demographics and clinical information
Of the 12 patients with TMA demonstrating the pattern of multifocal cortical and subcortical hemorrhagic infarctions, 8 (67%) had DIC (Fig 3). Three patients (25%) were diagnosed with mHTN. One patient (8%) had a diagnosis of TTP. This distribution of pathology reflects the disease range in the original cohort of 623 patients with TMA.
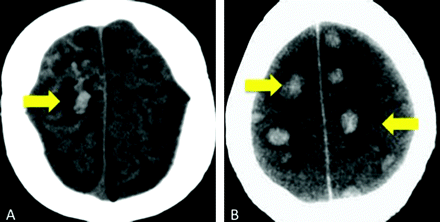
Cases 4 and 8. Axial noncontrast CT scans demonstrate peripheral hemorrhagic infarctions (arrows) in 2 patients with DIC.
Discussion
Injury to endothelial cells is the inciting factor in the sequence of events leading to TMA. The cascade of the microangiopathic process is the following: loss of physiologic thromboresistance, leukocyte adhesion to damaged endothelium, complement consumption, abnormal vWF release and fragmentation, and increased vascular shear stress.2 In TTP, thrombi formation occurs subsequent to the release of multimers of vWF. A specific plasma protease, the ADAMTS13 gene, is responsible for the physiologic degradation of vWF and plays a pathogenic role in a substantial proportion of familial and acute idiopathic cases of TTP.8 A deficiency in ADAMTS13 enzyme levels, along with an inhibitory antibody, is found in most patients with idiopathic TTP. Multiple triggers, such as infection, drugs, cancer, chemotherapy, bone marrow transplantation, and pregnancy, are recognized.9 In HUS, endothelial cell damage is considered the reason for complement and platelet activation leading to thrombus formation. Several complement genes are mutated in HUS.8
Multifocal TMA with intimal-medial dissection by thrombi extending from foci of endothelial damage in small cerebral arteries and arterioles can be secondary to toxins.10,11 A drug as the toxin is a rare cause of TMA.12 Antineoplastic therapy, the most reported agent being mitomycin, has been described as an etiology of renal TMA. The association of HIV and TMA is rare but well known; however, HIV-associated TMA is postulated to have a different pathophysiology than idiopathic TTP.13 Response to infection may generate antibodies that cross-react with platelet antigens. Platelet production may be impaired by infection of megakaryocyte bone marrow−dependent progenitor cells and decreased production of thrombopoietin.14 The classic toxin-induced TMA is HUS,11 in which directed or receptor-mediated verotoxin-induced endothelial injury leads to infarction and hemorrhage. Most studies of CNS involvement with HUS have described basal ganglia lesions.15–19 There are anecdotal reports of territorial infarction and diffuse white matter changes of PRES,18–21 but they are thought to reflect complications rather than specific manifestations of the disease. The pathophysiology of HUS may be, in part, due to blood-brain barrier breakdown and subsequent edema.18 Peripheral cortical involvement has not been described as a predominant imaging pattern in HUS.
Although there is a common pathophysiology in TMAs of microvascular occlusion due to endothelial injury and thrombus formation, the clinical findings can be varied, depending on the underlying disease. These patients may present clinically with altered mental status, seizure, or neurologic deficits. The suspicious neuroimaging of multifocal cortical and subcortical hemorrhagic infarctions in correlation with the underlying disease and laboratory findings enables the diagnosis of TMA. Neurologic manifestation of HUS may be due to hypoxia21 secondary to metabolic derangements, such as hyponatremia, azotemia, hydration disorders, or due to hypertension.11 As stated earlier, the incidence of TMA varies with the underlying diagnosis. Reviewing cases of TMA in our electronic data base for those with this particular imaging pattern showed a greater incidence in males with the most common diagnosis being DIC.
A systematic review of the literature was performed with a focus on those articles discussing TMA involving the CNS. In our retrospective review, DIC (82%) was the most common TMA.4 DIC is associated with many comorbidities including infection, neoplasm, vascular abnormalities, obstetrical and neonatal complications, massive tissue injury, and drug reactions. Clinically, DIC is typified by thrombosis and/or hemorrhage at multiple sites. Characteristic laboratory findings include thrombocytopenia, decreased fibrinogen, increased prothrombin time, and abnormalities in tests for fibrinolysis. In 1980, Buonanno et al7 described a series of 9 patients with DIC in whom venous thrombosis predominated as the most common imaging abnormality. The 2 patients with cross-sectional imaging had lobar hemorrhages.The second most common TMA in our retrospective review was mHTN. Patients with mHTN present with elevated blood pressure and papilledema, often with retinal hemorrhage and exudates.22 The most common reported neuroimaging findings are PRES, parenchymal hemorrhage, and infarctions. Garewal et al3 recently reported a case of mHTN presenting with extensive bilateral peripheral infarctions and hemorrhage with pathologic confirmation of a thrombotic microangiopathy. The least common TMA in our retrospective review, TTP, is characterized clinically by thrombocytopenia, microangiopathic hemolytic anemia, neurologic symptoms, fever, and renal dysfunction. This pentad of signs and symptoms is rarely present in its entirety, and often the triad of thrombocytopenia, elevated lactate dehydrogenase, and schistocytosis is sufficient to suggest the diagnosis.4 In a review of 12 patients with TTP, Bakshi et al6 described 3 imaging patterns: frank hematoma, ischemic infarctions, and reversible bilateral cerebral edema (similar to PRES). In a case report, D'Aprile et al23 described reversible multifocal gray matter edema. Gruber et al24 presented a case report of multiple small areas of cortical infarction. TTP may manifest as multiple punctate T2 hyperintensities in the cerebral white matter.25 Garewal et al3 described ischemic infarctions in the cortical and subcortical regions and PRES as the most characteristic neuroimaging finding in TTP.
There is possible parallel and/or overlap in the pathophysiology of TMA and PRES. One proposed mechanism of PRES is that severe hypertension leads to failed autoregulation and subsequent hyperperfusion with endothelial injury and vasogenic edema.25 The other hypothesized etiology is vasoconstriction and hypoperfusion resulting in brain ischemia and subsequent vasogenic edema.26 There are numerous pathologies, such as preeclampsia/eclampsia, allogenic bone marrow or organ transplantation, autoimmune disorders, and chemotherapy, recognized to produce the characteristic CT/MR imaging focal of regions of symmetric hemispheric edema, predominantly involving the parietal and occipital lobes.27 The basic pattern of PRES resembles watershed zones, with cortex and subcortical and deep white matter involvement to varying degrees. Histopathologic evidence of acute and chronic vessel injury has been described in postmortem studies, including intimal thickening, segmental vessel narrowing, intimal dissection, and organized thrombi.28 This supports a pathologic process similar to that of TMA. Minute hemorrhages as well as focal hematoma and sulcal hemorrhage can be seen with PRES.29 Excluding patients with allogenic bone marrow transplant, we noted no correlation between blood pressure and hemorrhage in PRES.29 Furthermore, Hefzy et al29 found no statistical significance in the coagulation state between hemorrhagic and nonhemorrhagic patients with PRES. The multifocal hemorrhages may be due to postischemic reperfusion injury.30 This differs in comparison with the subjects in our case series who had coagulopathy (ie, DIC and TTP) or elevated blood pressure (ie, mHTN).
Due to the retrospective nature of this work, there are several caveats. First, our cases have this distinct pattern of peripheral cortical and subcortical hemorrhagic infarction, and the diagnosis of TMA was confirmed with laboratory and clinical data. Biopsy was not feasible in these patients with coagulopathy. The small number of patients and the method of data acquisition (electronic data base and teaching files) preclude ascertaining a definite incidence of TMA in our case series. Minute hemorrhages can be absent on initial CT examinations.29 GRE and SWI sequences were not performed on every patient; therefore, subtle minute hemorrhages in a cortical and subcortical distribution may not have been detected on CT or conventional MR imaging. In patients with suspected TMA, the initial CT may be negative for hemorrhage; however, MR imaging can more sensitive for intraparenchymal hemorrhage, particularly echo-planar imaging−GRE T2*-weighted sequences.31 This increased MR imaging sensitivity for parenchymal hemorrhage is depicted in Fig 1. Even more advanced SWI sequences appear to be 3–6 times more sensitive than conventional T2*-weighted GRE sequences in detecting subtle minute hemorrhages.32,33 It is feasible, therefore, that patients with the particular imaging pattern described herein may not have been included. Last, our distribution of underlying disease is a reflection of our adult patient population. HUS is predominantly a childhood disease and was not seen in our referral base.
Conclusions
Despite a common underlying pathophysiology, TMAs demonstrate distinct clinical and laboratory features. Thus, the varied neuroimaging findings documented in the literature are not unexpected. Our series of 12 patients expands the imaging spectrum to include multifocal cortical and subcortical hemorrhagic infarctions. It is this pattern of peripheral cortical and subcortical hemorrhagic infarctions we describe that may prompt further investigation into the clinical diagnosis and alert the clinical service to the possibility of TMA.
References
- Received May 25, 2010.
- Accepted after revision August 25, 2010.
- Copyright © American Society of Neuroradiology