Abstract
BACKGROUND AND PURPOSE: Although MR imaging of the fetal brain has been shown to provide additional diagnostic information, the optimal timing of the study and the value of repeat studies remain unclear. The primary purpose of this study was to look for structural abnormalities of the fetal brain shown at 30–32 weeks' gestational age but not on the 20–24 weeks' study in fetuses originally referred with isolated VM. In particular, we wished to study the hypothesis that third-trimester fetal MR imaging studies would not show extra brain abnormalities compared with the second-trimester studies in this group.
MATERIALS AND METHODS: Ninety-nine women were admitted for a fetal MR study between 20–24 weeks' gestational age, and 46 of these women agreed to return for a second MR imaging examination at 30–32 weeks' gestational age. The other women were either lost to follow-up or declined the invitation to return. Two experienced observers measured the width of the trigones, and the results were compared, to test reliability. Changes in the degree of VM are reported along with changes in the diagnosis of structural brain abnormalities.
RESULTS: There was excellent reproducibility of trigone measurements between the 2 observers, with a mean absolute difference of <1 mm in the 40 fetuses that were ultimately shown to have isolated VM. Twenty-eight of 40 fetuses studied had mild VM on the first iuMR imaging examination, but in just more than half, the category of VM changed between the studies (5 had become normal-sized, 7 had progressed to moderate, 3 had become severe, and 13 remained mild). In 1 case, hypogenesis of the corpus callosum was recognized at 30–32 weeks but had not been reported on the 20–24 weeks' examination; the other 5 fetuses had brain pathology recognized on both fetal MR studies.
CONCLUSIONS: Trigone measurements can be made in a highly repeatable fashion on iuMR imaging. We have not shown any major advantage in repeating iuMR imaging at 30–32 weeks' gestation in terms of improved diagnosis of other structural brain abnormalities. With the converse of that argument, however, our data suggest that there is no advantage in delaying iuMR imaging studies to 30–32 weeks in the hope of improving detection rates.
Abbreviations
- iuMR imaging
- in utero MR imaging
- TEeff
- effective time to echo
- VM
- ventriculomegaly
- w
- weeks
Imaging of the fetal brain is routinely performed by using sonography, and measurement of the size of the lateral ventricles is an important part of the screening process.1,2 VM diagnosed in utero is found in 1%–2% of all pregnancies and is a common indication for more detailed fetal assessment. There is increasing evidence of the value of including iuMR imaging in the diagnostic pathway. Our recent work, for example, has shown that there is a 17% risk of finding brain abnormalities other than VM in fetuses referred from sonography with a confident diagnosis of isolated VM.3 That study included 147 singleton fetuses studied at 20 weeks' gestation or later, with the MR imaging being performed within a few days of the diagnosis of VM made on sonography. Subgroup analysis was made in that study on the basis of gestational age at referral. Ninety-nine of the cases studied were between 20 and 24 weeks' gestational age, and among these, there was a 9% pickup of extra brain pathology.3 As part of the secondary aims of that study, we invited all women from the 20 to 24 weeks' group to have a second iuMR imaging examination at 30–32 weeks' gestation if they continued with their pregnancy.
We have looked at changes in ventricular size during the 8–12 week period between the 2 MR imaging examinations and studied interobserver reproducibility of the trigone measurements on the MR imaging studies. The primary purpose of this study, however, was to look for structural abnormalities of the fetal brain shown at 30–32 weeks' gestational age but not on the 20–24 weeks' study in fetuses originally referred with isolated VM. In particular, we wished to study the hypothesis that third-trimester fetal MR imaging studies would not show extra brain abnormalities compared with the second-trimester studies in this group.
Materials and Methods
Participants
In the original study, women were recruited from 8 tertiary fetal assessment units in England and Scotland. As described in our earlier article,3 the entrance criteria for the study were the following: singleton pregnancy with fetal VM diagnosed on sonography (trigone measurement of ≥10 mm) referred to the study center at 20 weeks' gestational age or later (based on the sonography dating scan performed at 10–13 weeks) and no other abnormality of the fetus shown by sonography (brain or somatic) under good sonographic conditions. Although karyotyping had been performed on some fetuses before the iuMR imaging, the results were not known at the time of referral. The women did not have any known or suspected contraindications to MR imaging and agreed to provide written consent after full explanation by 1 of the authors. The women were not paid for their involvement in the study, but travel expenses were offered for themselves and a companion.
The subgroup described in this article consisted of 99 women recruited into the original study who had had iuMR imaging between 20 and 24 weeks' gestation. The original research proposal included follow-up iuMR imaging in women who had a first study at that stage if they continued with the pregnancy. Women in that arm of the study were told that they would be invited back for a second examination at 30–32 weeks' gestational age if they were agreeable to being approached. The referring fetomaternal center was contacted when the fetus was 28–29 weeks' gestational age to check that the pregnancy was ongoing. This was done to reduce the risk of an unnecessary approach to the woman if spontaneous or therapeutic abortion had occurred. The woman was then invited back for the repeat iuMR imaging.
Of the 99 women who were eligible to return for the second iuMR imaging examination, 90/99 (91%) had no other fetal brain abnormality diagnosed on the first iuMR imaging (isolated VM group). Three of those women had elected to terminate the pregnancy and were not recontacted. Two of those fetuses had severe and 1 had moderate VM. Forty-six women (51%) declined the second iuMR imaging examination or were otherwise lost to follow-up (39 fetuses with mild VM, 4 with moderate VM, and 3 with severe VM), leaving 41 women in this group.
Nine of 99 women (9%) had fetuses with brain abnormalities other than VM recognized on the first iuMR imaging. Of those 9 women, 2 elected to terminate the pregnancy and were not contacted and 2 women declined to return for a repeat examination, leaving 5 in this group.
Procedures
Our iuMR imaging technique has been described in detail elsewhere4 but is summarized here. All images were acquired at 1.5T scanner (either Infinion or HDx, GE Healthcare, Milwaukee, Wisconsin). Either a flexible phased array body coil or a cardiac coil was used, and a series of 3-plane scout views was obtained. After the fetal head was located, single-shot fast spin-echo sequences were run by using the following typical parameters: TR, 20,000 ms; TEeff, 75 ms; echo-train length, 132; FOV, 25 cm; matrix size, 248 × 256; NEX, 1; flip angle, 120°. Twenty 5-mm-thick sections of the fetal brain were obtained (18- to 25-second acquisitions) in the 3 natural orthogonal planes. After those were judged to be of diagnostic quality, similar acquisitions were performed by using 3-mm-thick sections with typical parameters: TR, 31,416 ms; TEeff, 92 ms; echo-train length, 136; FOV, 25 cm; matrix size, 183 × 256; NEX, 1; refocusing angle, 120°. An axial gradient-echo T1-weighted sequence was acquired in 30-second acquisition time, either with or without suspended maternal respiration. The parameters for the T1 sequence were TR, 238 ms; TE, 3.4 ms; refocusing angle, 70°; FOV, 23 cm; matrix size, 192 × 256; NEX, 1. At least 1 of the radiologists involved in the study (M.J.R., E.H.W., P.D.G.) was in attendance during the study to confirm that diagnostic-quality images had been obtained.
Analysis
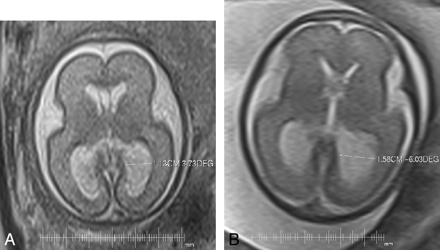
The size of the trigones of the lateral ventricles were determined initially on sonography by using a standardized axial view by the referring fetomaternal expert, and comparisons between the sonography and the MR imaging measurements were reported in our earlier article.3 For the purposes of the present article, the transverse diameters of the trigones of both lateral ventricles were obtained on axial iuMR imagings (Fig 1) of both the second- and third-trimester studies. The axial plane was chosen because in the previous study, sonography and MR imaging measurements of the trigones were compared, and in the United Kingdom, all trigone assessments are made in the axial plane. The trigone measurements on iuMR imaging were performed independently by 2 researchers experienced in iuMR imaging (J.E.M. and M.J.R.) to assess reproducibility. All of the measurements were made on proprietary software (Eclipse; Philips Healthcare or Advantage Workstation 4.4, GE Healthcare) and rounded to the nearest 1 mm (ie, with a precision of ±0.5 mm), because this corresponds to the most widely accepted clinical scale for categorization of severity (see below). The reviewers did not have access to either the measurements made on sonography or the measurement made on the other iuMR imaging study.
Two fetuses with different categories of VM, illustrating the measurements of the trigones of the lateral ventricles used in this study. A and B, Axial single-shot fast spin-echo images show mild (A) and severe (B) VM.
Interobserver agreement was tested both in terms of absolute measurement and categoric assessment of the degree of VM by using the following bounds: mild, 10–12 mm; moderate, 13–15 mm; and severe, ≥16 mm. These were based on the size of the larger of the 2 ventricles if the process was asymmetric.
The iuMR imagings were all reported clinically at the time of the examination, but for the purposes of this study, all were reviewed by radiologists experienced in iuMR imaging brain studies (P.D.G., M.J.R.) at a later date.
Any brain abnormalities other than VM were recorded. In those cases, the clinical significance of the extra findings made on the third-trimester iuMR imaging was assessed by 2 fetomaternal experts independently (G.M., S.A.R.).
Results
Isolated VM Group
Of the 41 women who did return for the second examination, 1 fetus was shown to have hypogenesis of the corpus callosum on the 30–32 weeks' study, despite it being reported as isolated VM on the 20–24 weeks' iuMR imaging study. That case was excluded from further analysis of ventricular size. Forty women, therefore, in this group had a fetus with isolated VM and underwent 2 iuMR imaging examinations. Reproducibility was assessed by comparing the trigone measurements made by 2 observers independently for all 40 women who had 2 iuMR imaging examinations for isolated fetal VM. In the 20–24 weeks' group, there was a mean absolute difference of <1 mm between the 2 observers in each case. This consisted of 40 complete agreements, 37 disagreements by 1 mm, and 3 disagreements by 2 mm, producing conflicting categoric classifications in 10/80 ventricles (8 between normal-sized ventricles and mild VM, 1 mild-versus-moderate, and 1 moderate-versus-severe). Even so, the overall classification (based on the largest ventricle) disagreed in only 2/40 cases (both being normal-versus-mild VM). In the 30–32 weeks' group, there was a mean absolute difference of <1 mm between the 2 observers per case. This consisted of 46 complete agreements, 33 disagreements by 1 mm, and 1 disagreement by 2 mm. This produced categoric disagreements in 5/80 ventricles (3 between normal-sized ventricles and mild VM, and 2 between mild and moderate), with the categoric classification conflicting in 3/40 cases (2 normal-versus-mild, 1 mild-versus-moderate VM).
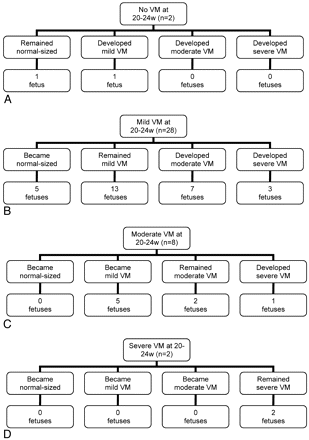
The ventricular sizes used in the following analysis were calculated as the mean of both iuMR imaging reviewers' measurements rounded to the nearest millimeter. The categoric description used in many sonography studies relates to the size of the larger of the 2 trigone measurements in the fetus (≤9 mm, normal; 10–12 mm, mild; 13–15 mm, moderate; and ≥16 mm, severe). With that approach, the degree of VM by iuMR imaging in the 20–24 weeks' group was the following: normal-sized, 2; mild VM, 28; moderate VM, 8; and severe VM, 2 cases. On the second iuMR imaging examination performed between 30 and 32 weeks, the overall categories of VM were the following: normal-sized, 6; mild VM, 19; moderate VM, 9; and severe VM, 6 cases. The changes in the degree of VM between 20–24 weeks' and 30–32 weeks' examinations, in terms of category, are shown in Fig 2. To summarize those, in 18/40 cases, the category remained unchanged; in 12/40, the category of VM increased; and in 10/40, the category decreased.
A−D, The change in category of VM between the 20–24 weeks' and the 30–32 weeks' iuMR imaging examinations is displayed on 4 charts in relation to the initial category of VM: normal-sized, mild, moderate, and severe.
Because the reproducibility studies showed that the difference of trigone measurements between the 2 observers was never >2 mm, we took a difference of mean trigone size of ≥3 mm to signify asymmetric VM. In the 20–24 weeks' group, 16/40 (40%) fetuses had asymmetric isolated VM. In 13 cases, this indicated unilateral VM (1 trigone measurement was normal; the other trigone was mild in 10 cases and moderate in 3 cases); and in 1 case, there was mild VM on 1 side and moderate VM on the other. There was also 1 case of mild-versus-severe VM, and 1 case in which the trigone measurements differed but both signified severe VM. In the 30–32 weeks' group, 17/40 had isolated asymmetric VM and 11 of those were unilateral VM (6, mild; 3, moderate; and 2, severe).
VM with Other Brain Abnormalities
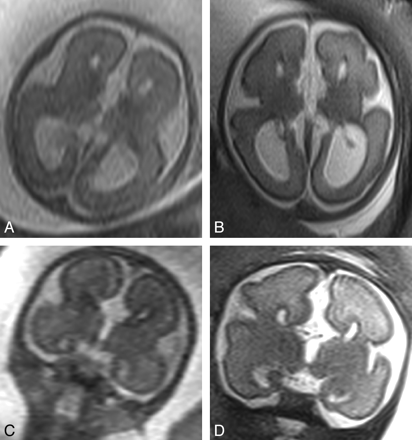
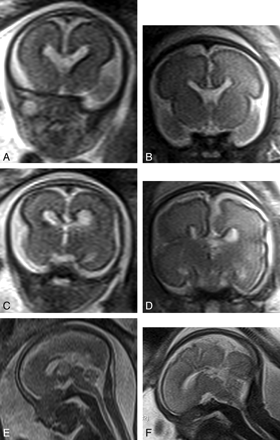
Of the 5 women who returned for a second iuMR imaging study, the fetal brain diagnoses based on the 20–24 weeks' iuMR imaging studies were the following: agenesis/hypogenesis of the corpus callosum (3 cases), absent cavum septum pellucidum (1 case), and cerebellar hypoplasia (1 case). None of those diagnoses were changed on the basis of the 30–32 weeks' iuMR imaging examination (examples are shown in Figs 3 and 4). As described above, there was also 1 case of hypogenesis of the corpus callosum that was detected on the 30–32 weeks' iuMR imaging examination but was not reported on the 20–24 weeks' iuMR imaging examination. Retrospective review of the 20–24 weeks' iuMR imaging study did show the callosal abnormality.
Appearances of a case of agenesis of the corpus callosum diagnosed on the 20–24 weeks' iuMR imaging examination (A and C) and reconfirmed on the 30–32 weeks' study (B and D). All images are from single-shot fast spin-echo sequences in the axial (A and B) and coronal (C and D) planes.
Appearances of a case of hypogenesis of the corpus callosum diagnosed on the 20–24 weeks' iuMR imaging examination (A, C, and E) and reconfirmed on the 30–32 weeks' study (B, D, and F). All images are from single-shot fast spin-echo sequences in the coronal plane anteriorly (A and B), the coronal plane posteriorly (C and D), and the sagittal plane (E and F). The images show that the genu and part of the body of the corpus callosum are present.
The reviewers of the clinical significance of this finding commented that a woman would almost certainly have been offered fetal karyotyping on the basis of the imaging at 20–24 weeks, but this was likely to have returned a normal result. As such, the clinician counseling her would have been incorrectly reassured by the original iuMR imaging findings. The finding of hypogenesis of the corpus callosum on the 30–32 weeks' iuMR imaging study would introduce far more uncertainty into the counseling process. Potential moderate-to-severe neurodevelopmental outcome would then have to be discussed along with the option of discussion with a neonatologist or pediatric neurologist. Regardless of the gestational age, the additional finding on the second iuMR imaging examination would normally mean that the issue of termination would also be discussed.
Discussion
The work of many imaging groups, including our own, has shown significant improvements in the prenatal diagnosis of brain abnormalities by using iuMR imaging alongside fetal neurosonography.3–8 There has been little or no attention paid to diagnostic errors made when interpreting iuMR imaging examinations, but at this stage, we cannot talk about the iuMR imaging findings in relation to a reference diagnosis based on postnatal follow-up. That work is planned but will include clinical and radiologic assessment when the children are 3–4 years of age, so it will not be available for some time. As an interim measure, we have presented the differences in the reports of the second- and third-trimester iuMR imaging examinations in this article. We set out to test the hypothesis that brain abnormalities other than VM would be obtained on a third-trimester iuMR imaging examination in at least 10% of cases. Our work, however, has shown that the extra pickup rate was considerably lower than that. In only 1 of 41 cases (2%, 95% confidence interval, 0%–13%, binomial exact method) assumed to have isolated VM on the 20–24 weeks' examination was a further brain abnormality shown on the 30–32 weeks' study (hypogenesis of the corpus callosum). The potential abnormality covers a range of severities, extending from merely absence of the rostrum through the presence of the genu only. Our case was from the more severe end of the spectrum (genu and anterior body present), and in retrospect, it was detectable on the first iuMR imaging study. It is clear from the retrospective review that in this case, the additional findings on the 30–32 weeks' iuMR imaging examination would have had a clinically significant impact on counseling and management options. In addition, we did not find any further diagnostic information on the 30–32 weeks' iuMR imaging examination in the 5 cases shown to have brain abnormalities on the 20–24 weeks' iuMR imaging studies.
Our provisional opinion, therefore, is that there is little to recommend the routine use of a third-trimester follow-up iuMR imaging study in cases of apparently isolated fetal VM if a good-quality study has been obtained at 20–24 weeks. The other side of the argument, however, is the important observation that there was no advantage in delaying iuMR imaging until 30–32 weeks as suggested in many parts of the published sonography literature.9 This is in contrast to sonography because the meta-analysis performed by Melchiorre et al9 showed a 12.8% extra pickup rate for follow-up sonography compared with the initial study. There are some situations in which further studies are warranted, such as when the 20–24 weeks' examination is equivocal or in a woman with a specific clinical or genetic concern about a cortical formation abnormality.
One limitation of this study, quite unexpected at the outset, was the relatively low take-up rate when repeat iuMR imaging was offered, with just under half of the women eligible returning for a third-trimester scan. We can only speculate on the reasons for this, and the effect it has on the study results. These women may have reached a firm decision on the management of their pregnancy based on their second-trimester investigations and consultation, so they did not consider a repeat examination necessary. Subsequent intervening antenatal sonography examinations may have been reassuring, signifying a good prognosis and discharge from specialist care, again implying that further examination was not required. In these circumstances, we might expect that many of the women who declined follow-up iuMR imaging would have had nonprogressive ventricular measurements and no additional findings on sonography, so our estimate of the rate of progressive VM may well be a little high. Without more specific study, however, this estimate cannot be confirmed, and there are many other complex factors to consider, which probably have a significant influence on any decision to re-attend. These include the distressing experience of antenatal investigation and diagnosis, the acceptability of MR imaging itself, and the difficulties of re-establishing contact with patients based on medical records.
Measurement of the trigones of the lateral ventricles is an important aspect of the routine anomaly scan.10,11 The importance of diagnosing fetuses with VM (trigone measurements ≥10 mm) has been reviewed extensively by Melchiorre et al,9 a work that contains many important meta-analyses. The cutoff of normality is <10 mm at any stage of pregnancy, and this is based on 2 large studies that showed a trigone measurement of 10 mm is between 3 and 4 standard deviations above the mean, assuming a normal distribution.1,2 With that classification, approximately 1% of pregnancies are complicated by VM.9 Our earlier article showed good agreement between trigone measurements made by different observers by using sonography and iuMR imaging. In this study, we have moved on to look at interobserver reproducibility of trigone measurements made on iuMR imaging. There was close agreement between our 2 observers, with no more than 1 mm difference measured in 77/80 trigones in the 20–24 weeks' group and 79/80 trigones in the 30–32 weeks' group. The importance of precise and accurate measurements of the trigone relates to the known risk of poor postnatal outcome. It is widely accepted that severe VM is defined by a fetal trigone measurement of >15 mm, but there is disagreement about the classification of VM of 10–15 mm. Many researchers and clinical practitioners use mild VM to describe any trigone measurement of 10–15 mm inclusive. Melchiorre et al advocated this approach and supported it with their meta-analysis finding that fetuses showing isolated VM of ≤12 mm do not have a statistically significant better neurologic prognosis compared with those of 12–15 mm. They describe a 16.6% risk of poor outcome in the 10–12 mm group and 11.8% in the 13–15 mm group. Other authors used “mild” to describe 10–12 mm trigones and “moderate” to describe 13–15 mm trigones.12 We used the latter classification because from an imaging viewpoint, we have found quite different risks of finding other brain abnormalities on iuMR imaging. Our recent report showed a risk of other brain abnormalities of 6% in supposed isolated mild VM and of 14% in moderate VM.3
There have been several sonography studies looking at the rate and significance of progressive fetal VM during pregnancy. Parilla et al13 used 10–15 mm as the definition of mild VM but presented their data that allow analysis of 10–12 mm and 13–15 mm subgroups separately. Overall 16% of 63 cases showed progression (ie, became severe), 43% stabilized (remained within 10- to 15-mm range), and 41% normalized (became <10 mm). Normalization was more commonly seen in the 10–12 mm group (25/47) compared with the 13–15 mm group (1/16), whereas progression was more equally distributed (7/47 and 3/16). We can compare our findings with those of Parilla et al by combining the data in Figs 2B and C. With this combination, our group of isolated VM cases with trigones between 10 and 15 mm showed stabilization in 27/36 (75%), normalization in 5/36 (14%), and progression in 4/36 (11%). The pooled analysis performed by Melchiorre et al9 also showed a progression rate of 16%, and in those cases, the developmental outcome was worse (44% rate of adverse outcome) than that in the nonprogressive cases (7% rate) with a relative risk of 6.32.
There are fewer published data concerning the rate and significance of asymmetry of the fetal ventricles. Our study showed a relatively high rate of unilateral VM, defined as 1 trigone ≥10 mm and the other ≤9 mm. In the 20–24 weeks' group, 33% (13/40) of cases had unilateral VM, and the rate in the 30–32 weeks' group was 28% (11/40). Unilateral fetal VM was thought to be quite unusual in the older sonography literature,14 but it must be remembered that in many cases, only the trigone furthest from the sonography probe is measured or measured with certainty, because of the problems of “near-field” effects.9 This was reflected in a recent sonography and iuMR imaging study in which unilateral VM was shown in 51/85 cases (60%) of fetuses with mild VM.15 There has been debate in the literature about the significance of unilateral isolated VM in the fetus in relation to bilateral VM. Melchiorre et al9 analyzed 5 published studies and came to the conclusion that there were no statistically significant differences in outcome in terms of neurodevelopmental delay (6% in unilateral VM and 7.4% in bilateral VM). Those conclusions should be predicated by the technical issues described above and by the low numbers from the pooled studies (approximately 100 cases).
Asymmetric VM in our study was defined as both trigones being ≥10 mm but with a difference of ≥3 mm between the 2 sides. This occurred in only 8% of cases (3/40) in the 20–24 weeks' group and 15% (6/40) of the 30–32 weeks' group. Melchoirre et al9 reviewed 3 published studies that discussed symmetry of VM. Using the definition of mild VM as 10–15 mm, fetuses with bilateral symmetric VM had a 4% risk of developmental delay, while bilateral asymmetric VM had poor outcome in 50%, but the numbers in this group were very small (4/8). Further research in the cases of unilateral VM and asymmetric VM supplemented by iuMR imaging is warranted.
Conclusions
We have not shown any advantage in repeating iuMRI at 30–32 weeks' gestation in cases of isolated fetal VM over and above the initial iuMR imaging findings at 20–24 weeks. In the 1 case in which there was another brain finding, the abnormality was detectable on the first examination on retrospective review. Conversely, these data suggest that there is no advantage in delaying iuMR imaging studies to 30–32 weeks in the hope of showing more pathology. Trigone measurements can be made in a highly repeatable fashion on iuMR imaging and should provide a good method to re-investigate the significance of changes in the degree of VM or the presence of unilateral VM in the future.
Footnotes

-
This work was supported by Westfield Health for the salary of Mike Reeves, MD, as a Clinical Research Fellow, and by the Wellcome Trust, United Kingdom. Elspeth Whitby, MD, was supported by the Health Foundation via the Academy of Medical Sciences.
-
The Wellcome Trust, Westfield Health, and The Health Foundation did not have any role in the design, in conducting this study, or in the preparation of this manuscript. The work was approved by the South Sheffield Research Ethics Committee.
Indicates open access to non-subscribers at www.ajnr.org
References
- Received March 3, 2010.
- Accepted after revision July 14, 2010.
- Copyright © American Society of Neuroradiology