Abstract
BACKGROUND AND PURPOSE: An ICH patient's risk of harboring an underlying vascular etiology varies according to baseline clinical and NCCT characteristics. Our aim was to develop a practical scoring system to stratify patients with ICH according to their risk of harboring a vascular etiology.
MATERIALS AND METHODS: Using a data base of 623 patients with ICH evaluated with MDCTA during a 9-year period, we developed a scoring system based on baseline clinical characteristics (age group [0–2 points], sex [0–1 point], neither known HTN nor impaired coagulation [0–1 point]), and NCCT categorization (0–2 points) to predict the risk of harboring a vascular lesion as the ICH etiology (SICH score). We subsequently applied the SICH score to a prospective cohort of 222 patients with ICH who presented to our emergency department during a 13-month period. Using ROC analysis, we calculated the AUC and MOP for the SICH score in both the retrospective and prospective patient cohorts separately and the entire patient population. Patients with SAH in the basal cisterns were excluded.
RESULTS: A vascular etiology was found in 120 of 845 patients with ICH evaluated with MDCTA (14.2%), most commonly AVMs (45.8%), aneurysms with purely intraparenchymal rupture (21.7%), and DVSTs (16.7%). The MOP was reached at a SICH score of >2, with the highest incidence of vascular ICH etiologies in patients with SICH scores of 3 (18.5%), 4 (39%), 5 (84.2%), and 6 (100%). There was no significant difference in the AUC between both patient cohorts (0.86–0.87).
CONCLUSIONS: The SICH score successfully predicts a given ICH patient's risk of harboring an underlying vascular etiology and could be used as a guide to select patients with ICH for neurovascular evaluation to exclude the presence of a vascular abnormality.
Abbreviations
- aPTT
- activated partial thromboplastin time
- AUC
- area under the curve
- AVF
- arteriovenous fistula
- AVM
- arteriovenous malformation
- CI
- confidence interval
- CTA
- CT angiogram
- DVST
- dural venous sinus thrombosis
- HTN
- hypertension
- ICH
- intracerebral hemorrhage
- INR
- international normalized ratio
- IVH
- intraventricular hemorrhage
- MDCTA
- multidetector CT angiography
- MIP
- maximum intensity projection
- MOP
- maximum operating point
- NCCT
- noncontrast CT
- ROC
- receiver operating characteristic analysis
- SAH
- subarachnoid hemorrhage
- SICH
- secondary ICH
Nontraumatic ICH accounts for 10%–15% of cases of acute stroke.1 Although in most cases the ICH is caused by hypertension, amyloid angiopathy, or impaired coagulation, historical features such as hypertension, older age, or coagulopathy, are often not enough, in isolation, to establish the ICH etiology. Indeed, in many cases, the ICH is due to vascular lesions such as AVMs, intracranial aneurysms with purely intraparenchymal rupture, and DVST (ie, SICH).1–3 Timely and accurate identification of patients with SICH is important because therapeutic options and rates of rehemorrhage are substantially different from primary ICH.1,2,4–6
Due to its widespread availability, rapidity of acquisition, lower cost, and favorable risk profile compared with conventional catheter angiography, MDCTA is rapidly becoming the favored diagnostic examination in the initial evaluation of patients presenting to the emergency department with ICH at many medical centers in the United States and around the world. In addition, several prior studies have demonstrated that compared with conventional catheter angiography and intraoperative findings, MDCTA is highly accurate for the detection of underlying vascular lesions in patients with ICH, with reported sensitivities ranging from 89% to 96% and specificities of 92% to 100%.7–10
A prior study of 623 patients with ICH examined with MDCTA at our institution identified clinical and NCCT characteristics that independently predicted an increased incidence of a vascular lesion as the ICH etiology: age younger than 46 years (47%), neither known hypertension nor impaired coagulation at presentation (33%), lobar (20%) or infratentorial (16%) ICH location, and female sex (18%).10 These findings are similar to those of several prior studies of patients with ICH examined with conventional catheter angiography.11–20 However, to date, no comprehensive system for the stratification of patients with ICH according to their risk of harboring an underlying vascular etiology has been developed. Indeed, the need for such a risk-stratification system and a standardized approach to the diagnostic work-up of this patient population is underscored by a recent literature review and multinational physician survey conducted at several leading medical institutions in Europe.21
The purpose of this study was to develop a practical scoring system to predict a given ICH patient's risk of harboring an underlying vascular lesion as the ICH etiology.
Materials and Methods
Patient Selection
Our study was approved by the institutional review board of our hospital and was conducted in compliance with the Health Insurance Portability and Accountability Act. First, we re-analyzed a retrospective cohort of 623 consecutive patients who presented to our emergency department from January 1, 2000, to November 1, 2008, with the following inclusion criteria: 1) age 18 years or older, 2) evidence of nontraumatic ICH on a NCCT examination of the head, and 3) evaluation with a CTA of the intracranial circulation within 24 hours of presentation. Subsequently, we conducted a prospective observational study of a cohort of patients who presented to our emergency department from November 2, 2008, to December 13, 2009, with the same inclusion criteria detailed above.
Patient exclusion criteria for both cohorts were the following: 1) the presence of associated SAH in the basal cisterns, 2) loss of gray-white matter differentiation in a vascular territory indicating a pre-established acute ischemic stroke, 3) a known intracranial vascular abnormality or mass lesion, or 4) known probable cerebral amyloid angiopathy according to the Boston criteria.22
Image Acquisition
NCCT and MDCTA examinations were performed according to standard protocols on 16- or 64-section helical CT scanners. The NCCT examination was performed by using an axial technique with 120–140 kVp, 170 mA, 2-second scanning time, and 5-mm-section-thickness reconstruction. MDCTA was subsequently performed by scanning from the base of the C1 vertebral body to the vertex by using the following parameters: pitch, 0.5; collimation, 1.25 mm; maximal mA, 350; kVp, 120; FOV, 22 cm; and 65–85 mL of nonionic contrast material administered by a power injector at 4–5 mL per second into an antecubital vein with either a fixed 25-second delay between the onset of contrast injection and the start of scanning (the delay was increased to 40 seconds in patients with atrial fibrillation) or SmartPrep, a semi-automatic contrast bolus triggering technique (GE Healthcare, Milwaukee, Wisconsin). All patients in the prospective cohort were scanned by using SmartPrep. The resulting 1.25-mm-thick axial source images were digitally archived. Standard MIP images of the major intracranial vessels were created by the 3D laboratory. The decision to perform MDCTA was at the discretion of the clinical providers.
Image Analysis
The image analysis methodology in the retrospective patient cohort has been previously published.10 In the prospective patient cohort, the NCCTs were reviewed by 2 neuroradiologists blinded to the clinical data and final diagnosis, to determine the presence of associated SAH and NCCT categorization as detailed below. All differences in reader interpretations for the NCCT categorization were resolved by consensus. At least 2 weeks after review of the NCCT, the CTA source and MIP images were reviewed by the same 2 neuroradiologists, blinded to the NCCT categorization, clinical data, and final diagnosis, to determine, by consensus reading, the presence of an underlying vascular etiology for the ICH.
A high-probability NCCT was defined as an examination in which there were either 1) enlarged vessels or calcifications along the margins of the ICH or 2) hyperattenuation within a dural venous sinus or cortical vein along the presumed venous drainage path of the ICH. A low-probability NCCT was defined as an examination in which none of the findings of a high-probability NCCT were present and the ICH was located within the basal ganglia, thalamus, or brain stem. An indeterminate NCCT was defined as an examination that did not meet criteria for a high- or low-probability NCCT (most commonly, lobar or cerebellar ICH).
A positive CTA was defined as a study in which an underlying vascular lesion as the ICH etiology was identified. For the diagnosis of DVST and/or cortical vein thrombosis, nonopacification of the affected venous structure on the first-pass CTA was confirmed by either persistent nonopacification on delayed images (if obtained) or corresponding hyperattenuation in the NCCT examination.
Medical Record Review
Medical records were reviewed for patient age, sex, known hypertension, and impaired coagulation. Patients were classified as hypertensive if they had a known history of hypertension or were taking antihypertensive medications at presentation. Patients were classified as having impaired coagulation if, at presentation, they were receiving daily antiplatelet therapy with aspirin or clopidogrel, had a platelet count of <50,000 cells per cubic mm of blood, had an INR of >3.0,23 or had an aPTT of >80 seconds.
Statistical Analysis
Statistical analysis was performed using the MedCalc package for Windows, Version 11.1 (MedCalc Software, Mariakerke, Belgium). First, we re-analyzed our previously reported retrospective cohort of 623 patients with ICH to include both the ICH location and NCCT categorization in addition to the baseline clinical characteristics in the multivariate logistic regression model to determine the independent predictors of a positive CTA.
Subsequently, we used the independent predictors of a positive CTA to construct a scoring system to predict a given ICH patient's risk of harboring a vascular lesion as the ICH etiology. We then applied this scoring system to both the retrospective derivation and prospective validation patient cohorts and performed ROC analysis to determine the AUC and MOP for the scoring system in both cohorts separately and in the entire patient population. A P value ≤ .05 was considered statistically significant.
Results
The baseline clinical and radiologic characteristics of the 623 patients included in our retrospective cohort have been published previously.10 From November 2, 2008, to December 13, 2009, a total of 253 adult patients presented to our emergency department with ICH on an NCCT examination and were evaluated with a CTA of the intracranial circulation within 24 hours of presentation. Thirty-one patients were excluded from the study (12.3%): 14 due to the presence of associated SAH within the basal cisterns (9 of which had an underlying vascular etiology [64.3%]), 8 due to a known vascular or mass lesion, 7 due to loss of gray-white matter differentiation in a vascular territory indicating a pre-established acute ischemic stroke, and 2 due to known amyloid angiopathy.
Hence, a total of 222 patients met the inclusion criteria of the prospective cohort, with a mean age of 67 years (range, 18–94 years), 111 of which were men (50%) and 111, women (50%). The number of patients with ICH evaluated with MDCTA constituted approximately 50.8% of the total number of patients with nontraumatic ICH presenting to our emergency department during the time period of the retrospective cohort and 77.8% of the patients with nontraumatic ICH presenting to our emergency department during to the time period of the prospective cohort.
Vascular ICH Etiologies in the Patient Population
Table 1 depicts the frequency of the different vascular ICH etiologies in both patient cohorts separately as well as in the entire patient population. MDCTA demonstrated a vascular ICH etiology in 29 of the 222 patients included in the prospective patient cohort (13.1%). Overall, an underlying vascular etiology for the ICH was identified in 120 of the 845 patients in the entire patient population (14.2%).
Vascular ICH etiologies identified by MDCTA
In the prospective patient cohort, the most common vascular etiologies for the ICH were AVMs (15, 51.7%; Fig 1), aneurysms with purely intraparenchymal rupture (5, 17.2%; Fig 2), DVSTs (3, 10.3%; Fig 3), and AVFs (3, 10.3%; Fig 4). In the entire patient cohort, the most common vascular ICH etiologies were AVMs (55, 45.8%), aneurysms with purely intraparenchymal rupture (26, 21.7%), DVSTs (20, 16.7%), and AVFs (11, 9.2%). However, in a small number of patients, the ICHs were secondary to vasculopathy (4, 3.3%; Fig 5) and Moyamoya phenomenon (4, 3.3%; Fig 6).
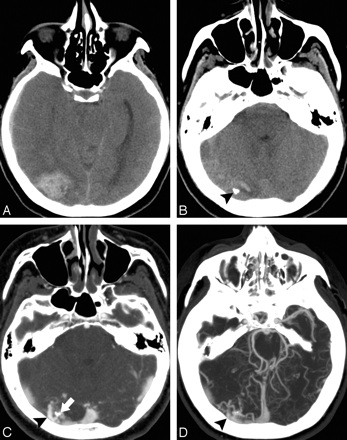
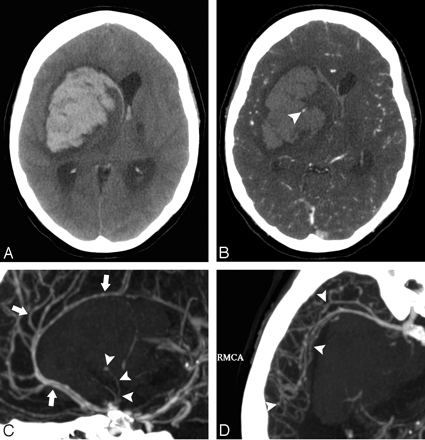
A 45-year-old woman without a history of hypertension and with intact coagulation presented with acute onset of headache and visual changes. A and B, High-probability NCCT scan demonstrates an acute right occipital ICH with calcifications along its posteroinferior margin (arrowhead, B; SICH score, 6). There was associated subdural hemorrhage overlying the right temporal lobe but no associated IVH or SAH. C, CTA source image demonstrates a tangle of abnormal vessels along the posteroinferior aspect of the ICH (arrowhead) with associated calcifications (arrow), consistent with an AVM. D, CTA MIP image in the axial plane redemonstrates the right occipital AVM (arrowhead) with arterial supply from branches of the right posterior cerebral artery and drainage to the right transverse sinus.
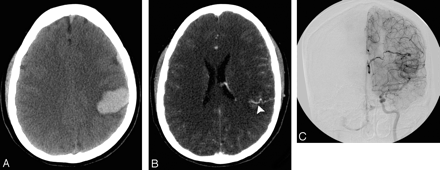
A 50-year-old woman with a history of hypertension and intact coagulation presented with acute onset of left-sided weakness. A, Indeterminate NCCT scan demonstrates an acute right temporal ICH without associated IVH or SAH (SICH score, 3). B, CTA source image demonstrates an 11-mm outpouching arising from the right MCA bifurcation (arrowhead), consistent with an aneurysm. C, CTA MIP image in the axial plane redemonstrates the right MCA bifurcation aneurysm (arrowhead).
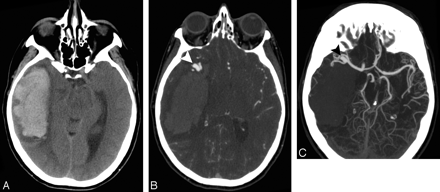
A 60-year-old woman without a history of hypertension and with intact coagulation presented with increasing headache during the past several days. A and B, High-probability NCCT scan demonstrates an acute right mesiotemporal ICH with subtle associated hyperattenuation within the distal right vein of Labbe (arrow, B) and right transverse sinus (arrowhead, B; SICH score,5). C, Coronal NCCT scan reformation improves depiction of the hyperattenuation within the distal right vein of Labbe (arrow) and right transverse sinus (arrowhead). D, CT venogram source image obtained immediately after the CTA demonstrates nonopacification of the right transverse and sigmoid sinuses (arrowheads), consistent with DVST. E, CT venogram MIP image after calvarial segmentation redemonstrates the right transverse and sigmoid sinus thrombosis (arrowheads).
A 44-year-old woman without history of hypertension and with intact coagulation presented with headache. A, Indeterminate NCCT scan demonstrates a left parietal ICH (SICH score, 5). There was associated subdural hemorrhage overlying the left frontal lobe but no associated IVH or SAH. B, CTA source image demonstrates an abnormal vessel along the inferior aspect of the ICH in the left parietal lobe, consistent with an AVF (arrowhead). C, Frontal left internal carotid artery catheter angiogram confirms the presence of a left parietal AVF with deep venous drainage into the left internal cerebral vein.
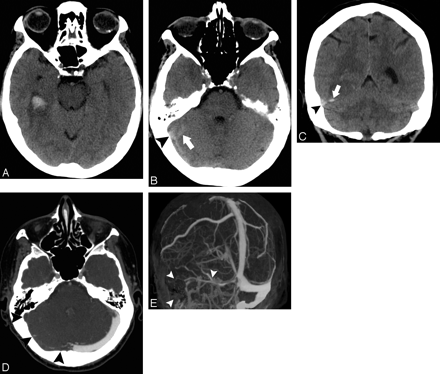
A 50-year-old woman without a history of hypertension and with intact coagulation presented with acute onset of unresponsiveness. A, Low-probability NCCT scan demonstrates an acute right basal ganglia ICH with associated IVH (SICH score, 3). B, CTA source image demonstrates a 3-mm outpouching arising from a lenticulostriate branch of the right middle cerebral artery (arrowhead), consistent with an aneurysm. C, CTA MIP image in the sagittal plane redemonstrates the right lenticulostriate aneurysm (arrowheads) as well as a diffuse luminal irregularity in the visualized anterior cerebral artery branches (arrows). D, CTA MIP image in the axial plane demonstrates diffuse luminal irregularity in the right middle cerebral artery branches (arrowheads). These findings are consistent with vasculitis with secondary pseudoaneurysm formation and rupture. The patient was ultimately found to have Lyme disease affecting the central nervous system.
A 42-year-old woman without a history of hypertension and with intact coagulation presented with severe headache. A, Indeterminate NCCT scan demonstrates an acute right temporal ICH without associated IVH or SAH (SICH score, 5). B, CTA source image demonstrates occlusion of the supraclinoid segments of the internal carotid arteries and proximal M1 segments of the middle cerebral arteries bilaterally, with numerous associated lenticulostriate collateral vessels (arrowheads), consistent with Moyamoya phenomenon. C, CTA MIP image in the axial plane redemonstrates the findings of Moyamoya phenomenon (arrowheads).
NCCT Categorization
Interobserver agreement for the NCCT categorization in the prospective cohort was almost perfect (κ statistic, 0.97; 95% CI, 0.93–1.0). In this cohort, 80 NCCTs were categorized as low-probability (36%), 2 of which had a positive CTA (2.5%, 1 lenticulostriate aneurysm and 1 lenticulostriate pseudoaneurysm secondary to vasculitis); 132 NCCTs were categorized as indeterminate (59.5%), 19 of which had a positive CTA (14.4%); and 10 NCCTs were categorized as high-probability (4.5%, 8 due to enlarged vessels or calcifications along the margins of the ICH and 2 due to hyperattenuation within a dural venous sinus or cortical vein along the presumed venous drainage path of the ICH), 8 of which had a positive CTA (80%).
In the entire patient population, 263 NCCTs were categorized as low-probability (31.1%), 6 of which had a positive CTA (2.3%, 2 basal ganglia AVMs, 1 thalamic AVM, 1 pontine AVM, 1 lenticulostriate aneurysm, and 1 lenticulostriate pseudoaneurysm secondary to vasculitis); 553 NCCTs were categorized as indeterminate (65.5%), 90 of which had a positive CTA (16.3%); and 29 NCCTs were categorized as high-probability (3.4%, 16 due to enlarged vessels or calcifications along the margins of the ICH and 13 due to hyperattenuation within a dural venous sinus or cortical vein along the presumed venous drainage path of the ICH), 24 of which had a positive CTA (82.8%).
Re-Analysis of the Multivariate Logistic Regression Model in the Retrospective Cohort
When the NCCT categorization was added to the previously published multivariate logistic regression model that included the ICH location and baseline clinical characteristics (age group, sex, known hypertension, impaired coagulation, and neither known hypertension nor impaired coagulation at presentation), the following variables became the independent predictors of a positive CTA: age group (P value < .0001), NCCT categorization (P value < .0001), neither known hypertension nor impaired coagulation at presentation (P value = .014), and sex (P value = .032).
The SICH Score
We used the independent predictors of a positive CTA—age group, NCCT categorization, neither known hypertension nor impaired coagulation at presentation, and sex—to construct a practical scoring system to predict a given ICH patient's risk of harboring a vascular lesion as the ICH etiology and designated it the SICH score (Table 2).
Calculation of the SICH score
The results of the application of this scoring system to both the retrospective-derivation and prospective-validation patient cohorts, as well as to the entire patient population, are shown in Table 3. The SICH score successfully predicted a given ICH patient's risk of harboring a vascular etiology in each patient cohort separately as well as in the entire patient population, with the highest incidences of vascular lesions observed in patients with SICH scores of 3 (18.5%), 4 (39%), 5 (84.2%), and 6 (100%).
Predictive value of the SICH score
In ROC analysis, there was no significant difference in the AUCs between the retrospective (0.86; 95% CI, 0.83–0.89) and prospective patient cohorts (0.87; 95% CI, 0.82–0.91). In addition, the MOP was reached at a SICH score of >2 in both patient cohorts as well as in the entire population, with high sensitivity (85.8%) and specificity (72.3%) for the detection of the vascular ICH etiologies present in the entire patient population.
Overall, patients with low SICH scores (0–2) comprised 64% of patients in our entire population and had a low incidence of vascular etiologies (3.1%), whereas patients with high SICH scores (≥3) comprised 36% of patients and had a high incidence of vascular etiologies (33.9%).
Discussion
We have developed and prospectively validated a practical scoring system that predicts a given ICH patient's risk of harboring an underlying vascular etiology, the SICH score. The practicality of the SICH score lies in its ease of calculation because it requires only review of the NCCT to determine its categorization (high probability, indeterminate, low probability) and clinical data routinely obtained on presentation to the emergency department (patient age, sex, history of hypertension, use of antiplatelet medications, platelet count, INR, and aPTT).
The SICH score can be rapidly calculated immediately after the NCCT examination has been performed while the patient is still on the CT scanner table, and could serve as a valuable tool in the clinical decision of whether to perform neurovascular evaluation. Therefore, the SICH score may be most useful at institutions in which neurovascular evaluation is not routinely performed on all patients with ICH but is reserved for those patients who are deemed most likely to harbor an underlying vascular abnormality. For example, at these institutions, patients with a SICH score of zero would not merit neurovascular evaluation, patients with SICH scores of 1–2 could be initially screened with a noninvasive technique such as MDCTA and would only undergo further evaluation with conventional catheter angiography if the initial CTA is either positive or equivocal, and patients with SICH scores ≥3 could be evaluated directly with conventional catheter angiography.
However, some institutions (including our own) consider a positivity rate of >10% for a low-risk noninvasive diagnostic test such as MDCTA to be high enough to merit performing it in all patients. Furthermore, at our institution (and others around the world), the presence of a “spot sign” is increasingly being used as a valuable tool for the prediction of hematoma expansion and poor clinical outcome in patients with primary ICH.24-32 Hence, the usefulness of the SICH score at institutions like our own may lie in selecting patients with ICH for more invasive diagnostic tests such as conventional catheter angiography when the initial MDCTA examination is either negative or equivocal. For example, at these institutions, patients with low SICH scores (0–2) would only merit further evaluation with conventional catheter angiography if the initial CTA is either positive or equivocal, while patients with high SICH scores (≥3) would merit further evaluation with conventional catheter angiography even if the initial CTA is negative.
The diagnostic approaches proposed above would minimize the overall number of negative conventional catheter angiograms performed in this patient population while also minimizing the number of patients with underlying vascular abnormalities who are not diagnosed correctly. This, in turn, would result in decreased radiation exposure in this patient population as well as reduced exposure to iodinated contrast material in patients with ICH and renal insufficiency or failure.
The limitations of the our study are the retrospective nature of the derivation cohort of the SICH score, the potential selection bias generated by the inclusion of only patients who presented with ICH and were evaluated with MDCTA, and the lack of independent validation of this scoring system. Although currently at our institution, most patients with nontraumatic ICH are evaluated with MDCTA on admission to assess for the presence of an underlying vascular etiology as well as a “spot sign,” in the retrospective cohort only approximately half of patients with nontraumatic ICH were evaluated with MDCTA on admission because patients who, on clinical grounds, were deemed unlikely to harbor an underlying vascular etiology for the ICH (likely those with low SICH scores) did not undergo MDCTA evaluation. However, the similar CTA positivity rates in each SICH score group and the similar AUCs for the scoring system in both patient cohorts provide evidence that this selection bias may not be significant. Nevertheless, the incidence of underlying vascular etiologies in our patient population, particularly in patients with low SICH scores, may be lower than reported in this study.
Conclusions
The SICH score successfully predicts a given ICH patient's risk of harboring an underlying vascular etiology. This practical scoring system could be used as a guide for selecting patients with ICH for neurovascular evaluation to exclude the presence of a vascular abnormality as the ICH etiology. Independent validation of this scoring system is necessary.
Acknowledgments
We thank Eleni K. Balasalle for her contribution to the artwork for this manuscript.
Footnotes
-
Indicates Editor's Choices selection
-

-
The following sources of funding were used, in part, for the preparation of this manuscript: American Heart Association Grant-in-Aid 0755984T and the National Institutes of Health/National Institute of Neurological Disorders and Stroke grant K23NS059774.
-
Paper previously presented in part at: International Stroke Conference, February 24, 2010; San Antonio, Texas; and as a whole at: Annual Meeting of the American Society of Neuroradiology, May 20, 2010; Boston, Massachusetts.
Indicates open access to non-subscribers at www.ajnr.org
References
- Received February 11, 2010.
- Accepted after revision April 4, 2010.
- Copyright © American Society of Neuroradiology