Abstract
SUMMARY: Mutations of the CASK gene are associated with X-linked mental retardation with microcephaly and disproportionate brain stem and cerebellar hypoplasia in females. The areas of the cerebrum, corpus callosum, pons, midbrain, and cerebellar vermis and hemisphere and a ratio of cerebrum/corpus callosum areas were measured in 5 female patients with CASK mutations, 67 female controls, and 5 patients with pontine hypoplasia. MR imaging in patients with CASK mutations revealed a normal size of the corpus callosum and a low ratio of the cerebrum/corpus callosum with a reduced area of the cerebrum, pons, midbrain, and cerebellar vermis and hemispheres. The 5 patients with pontine hypoplasia showed thinning of the corpus callosum and a high ratio of the cerebrum/corpus callosum, irrespective of the size of the cerebrum. The normal size of the corpus callosum, which gives an impression of callosal thickening at first glance, may be an imaging clue to detect patients with CASK mutations.
Abbreviations
- CASK
- calcium/calmodulin-dependent serine protein kinase
- CINAP
- CASK interacting nucleosome assembly protein
- PEHO
- progressive encephalopathy, edema, hypsarrhythmia, and optic atrophy
- TBR1
- T-brain-1
- RELN
- reelin
Advances in MR imaging and molecular biology have revolutionized the analysis and understanding of malformations of the brain. A classification of malformations of the midbrain and hindbrain has been proposed on the basis of embryology and genetics,1 dividing these disorders into 4 groups: 1) malformations secondary to early anteroposterior and dorsoventral patterning defects or to misspecification of midhindbrain germinal zones, 2) malformations associated with later generalized developmental disorders that significantly affect the brain stem and cerebellum, 3) localized brain malformations that significantly affect the brain stem and cerebellum, and 4) combined hypoplasia and atrophy in putative prenatal-onset degenerative disorders.1
Mutations of the CASK gene at Xp11.4 have recently been reported to have a wide phenotypic spectrum, ranging from a severe form in female patients (mental retardation and microcephaly with disproportionate brain stem and cerebellar hypoplasia)2,3 to a milder form in male patients with congenital nystagmus and mental retardation.4 The severe form of CASK mutations has been classified in midbrain-hindbrain classification group II.C.3, microcephaly with severe and disproportionate brain stem and cerebellar hypoplasia.1 The striking difference in clinical severity between the 2 groups of CASK mutations is explained by genotype-phenotype variability—that is, hemizygous missense mutations in males are likely less severe than inactivating mutations (causing more severe neurologic disability in females and prenatal or neonatal lethality in males).4 Male patients with the mild form of CASK mutations rarely have microcephaly or cerebellar hypoplasia and would be difficult to diagnose by neuroimaging. Patients with severe CASK mutations, however, might be detectable among girls with mid-hindbrain hypoplasia of unknown cause on MR imaging. We report herein MR imaging findings with volumetric data in 5 female patients with CASK mutations to determine whether they have characteristic imaging findings compared with other patients with pontine hypoplasia.
Case Series
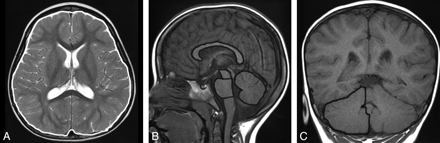
Five Japanese girls, 1–4 years of age, presented for medical assessment with histories of developmental retardation, microcephaly, and characteristic facial appearances (large pupils, large ears, and small jaw); these features led us to search for CASK mutations, which were found for all the 5 patients by array−comparative genomic hybridization and mutation analysis of the CASK gene by using the same methods previously reported.3 Details of the clinical and genetic information are under preparation for a report elsewhere. MR imaging was performed at 1.5T. Patient age at the time of imaging ranged between 9 and 50 months. One patient was scanned twice at ages 9 and 24 months; therefore, a total of 6 scans were analyzed. The areas of the pons, midbrain tegmentum, cerebellar vermis, and corpus callosum were measured in each patient on a midsagittal image (Fig 1 B). The area of the cerebrum was measured on a transverse image through the basal ganglia (Fig 1A), while that of the cerebellar hemispheres (an average area of both cerebellar hemispheres, including parts of the cerebellar peduncles) was measured on a coronal image through the fourth ventricle and nodulus of the vermis (Fig 1C). These measurements were performed on a NCC-CIR viewer (IBM, Armonk, New York) by 1 pediatric neurologist (J.T.). Written informed consent for genetic and clinical analysis was obtained from the parents after institutional review board approval was obtained from Tokyo Medical and Dental University and Kameda Medical Center.
MR imaging of a 3-year-old girl with febrile seizures showing (outlined in white) areas for the cerebrum on a transverse image (A); corpus callosum, pons, midbrain tegmentum, and cerebellar vermis on the sagittal image (B); and the right cerebellar hemisphere on the coronal image (C).
For comparison, the areas of these same regions were measured in 67 female patients (0.5–180 months of age) evaluated at Kameda Medical Center for mild neurologic symptoms, such as headache, hypotonia, seizures, febrile delirium, or mild asphyxia. No parenchymal lesions were apparent on MR imaging, the patients had no genetic abnormalities or syndromes, and no abnormalities were detected on subsequent neurodevelopmental examinations. In addition, the measurements of the patients with CASK mutations were compared with those of 5 patients with pontine hypoplasia due to causes other than CASK mutations. One of these patients was a previously reported male patient with PEHO syndrome, who was imaged at 16 and 111 months of age.5 The others consisted of a previously reported 17-month-old girl with 5p− syndrome,6 an 8-month-old boy with trisomy of chromosome 18, a 45-month-old girl with 46,XX,der(6)(qter→p25 ::q22.2→qter), and a 138-month-old girl with complex chromosomal abnormalities (normal X chromosome). The CASK gene was not analyzed in these 5 patients.
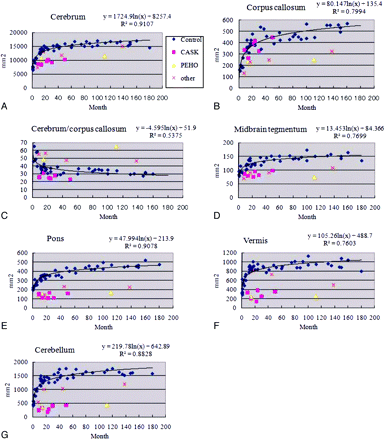
The areas of the cerebrum, corpus callosum, pons, midbrain tegmentum, cerebellar hemisphere, and vermis and a ratio of the cerebrum/corpus callosum areas in the controls and patients are shown in Figs 2 and 3. Areas of all regions in the controls increased with age, and the cerebrum/corpus callosum ratio decreased with age, reaching an adult value at around 5 years of age. Areas of the cerebrum, pons, cerebellar hemisphere, and vermis in the girls with CASK mutations were much reduced in size even in infancy (Figs 2B and 3) and showed little size increase with age. The midline sagittal area of the midbrain tegmentum was in the low-normal range in the patient with the CASK mutation imaged at 9 months of age (pink squares in Fig 3D) and showed little change on the second MR imaging at 24 months of age, making it obviously small compared with that in the controls, as observed in the other 4 patients with CASK mutations. The midline corpus callosum area was within the normal range in all 5 patients with CASK mutations (Figs 2B and 3B), leading to an impression of thickening compared with the small cerebrum. The callosal area increased normally with increasing age in the patient scanned twice (at 9 and 24 months of age). The cerebellum/corpus callosum ratio was low-normal or low in all patients with CASK mutations (Fig 3C). No obvious malformations were seen in the cerebral hemispheres of patients with CASK mutations. The MR imaging of the patients with PEHO (Fig 2C) and trisomy 18 showed reduced size in all examined regions. The other 3 patients with chromosomal abnormalities and pontine hypoplasia had a reduced size of the pons, midbrain, and corpus callosum with normal-to-small cerebral and cerebellar areas. Thus, the corpus callosum was always reduced in size in the 5 patients with non-CASK-related pontine hypoplasia; all had a high cerebrum/corpus callosum ratio except for the 8-month-old patient with trisomy 18.
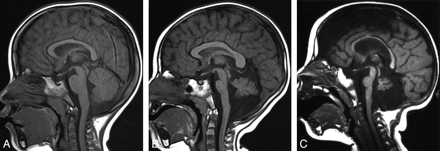
Comparison of sagittal images. A, T1-weighted sagittal image of a 28-month-old control girl. B, T1-weighted sagittal image of a 24-month-old girl with CASK mutations. Note the hypoplastic pons, midbrain tegmentum, and cerebellar vermis with normal appearance of the corpus callosum. C, Sagittal T1-weighted image of a 16-month-old patient with PEHO syndrome shows hypoplasia of all visible structures.
Longitudinal changes of the area of the cerebrum (A), corpus callosum (B), midbrain tegmentum (D), pons (E), cerebellar vermis (F), cerebellar hemisphere (G), and ratio of the cerebrum/corpus callosum (C). Blue diamonds represent control patients, pink squares represent patients with CASK mutations, yellow triangles represent the patient with PEHO syndrome, and the red x represents the patients with other midbrain-hindbrain malformations. Logarithmic regression curve for the controls in the cerebrum, y = 1724.9ln(x) + 8257.4, R2 = 0.9107; corpus callosum, y = 80.147ln(x) + 135.4, R2 = 0.7994; midbrain tegmentum, y = 13.453ln(x) + 84.366, R2 = 0.7699; pons, y = 47.994ln(x) + 213.9, R2 = 0.9078; vermis, y = 105.26ln(x) + 488.7, R2 = 0.7603; cerebellar hemisphere, y = 219.78ln(x) + 642.89, R2 = 0.8828; and ratio of cerebrum/corpus callosum, y = −4.595ln(x) + 51.9, R2 = 0.5375.
Discussion
The most important outcome in this study is that MR imaging findings of mid-hindbrain hypoplasia and a normal- or large-appearing corpus callosum in a girl with microcephaly and neurodevelopmental retardation should suggest the possibility of a CASK mutation, particularly if the cerebrum/corpus callosum ratio is low.
CASK belongs to the membrane-associated guanylate kinase protein family, functioning as a multidomain scaffolding protein and has an important function during neuronal development.2,7,8 CASK regulates gene expression by interacting with CINAP and the transcription factor TBR1. These proteins form a complex that induces transcription of genes containing TBR1 binding sequences, such as RELN.8 The CASK-TBR1-RELN cascade is required for normal development of the cerebrum, brain stem, and cerebellum. Loss of Cask or Tbr1 in mouse models results in microcephaly2,9,10; loss of Cask or Reln results in brain stem hypoplasia and defective inward migration of granule cells of the cerebellum.2,11 In humans, mutations in RELN are associated with lissencephaly and profound cerebellar hypoplasia.12 Inactivating mutations of CASK in humans have recently been reported to be associated with microcephaly and midhindbrain hypoplasia.2
Microcephaly with a small cerebrum, pons, midbrain, and cerebellum, as observed in this study, is compatible with the imaging findings described in a previous report2; however, the size of the corpus callosum was not previously mentioned, except for 1 severely affected male neonate whose MR imaging showed thinning of the corpus callosum. This child died at 2 weeks of age. Midsagittal images of the 4 previously reported female patients with CASK mutations showed a relatively normal appearance of the corpus callosum,2 supporting our findings. The 5 other patients in this study with (non-CASK-associated) pontine hypoplasia all had thinning of the corpus callosum and a high cerebrum/corpus callosum ratio, irrespective of the size of the cerebrum. MR imaging studies of patients with pontocerebellar hypoplasia types 1–3 also show progressive thinning of the corpus callosum.13 The apparent enlargement of the (normal-sized) corpus callosum, when viewing the midline sagittal MR image, may, therefore, be an imaging clue for detecting patients with CASK mutations when seen in the setting of microcephaly and neurodevelopmental retardation with midhindbrain hypoplasia.
Neuropathology of CASK mutations has been reported in a single male patient who died at 2 weeks of age.2 The pons was markedly reduced in size because of the loss of neurons in the basis pontis. The cerebellum showed poorly formed, shallow, and unbranched folia; hypercellularity in the molecular layer and increased thickness of the external granular layer with hypoplasia or absence of the internal granular layer and Purkinje cells. The frontal cortex was moderately disorganized and mildly thickened. Cerebral cortical laminae I-IV appeared normal, but layers V and VI merged together and showed a vaguely nodular organization; these layers subtly merged into the cerebral white matter. Reflecting the pathology, MR imaging of this male patient showed frontal pachygyria and pontocerebellar hypoplasia. MR imaging of the 4 female patients previously reported with CASK mutations showed microcephaly with a simplified gyral pattern.2 MR imaging of the 5 patients reported here, however, showed normal sulcal depth with an almost normal number and complexity of gyri. This imaging discrepancy might be explained by genotype-phenotype variability of CASK mutations. Further clinical and pathologic studies, possibly including imaging studies with a high magnetic field or high-resolution images, will be necessary to reach a definite conclusion.
Conclusions
CASK mutations are associated with X-linked mental retardation and microcephaly with disproportionate brain stem and cerebellar hypoplasia in females. MR imaging in 5 female patients with CASK mutations revealed a normal size of the corpus callosum and a low ratio of the cerebrum/corpus callosum, with a reduced area of the cerebrum, pons, midbrain, and cerebellar vermis and hemispheres. The normal size of the corpus callosum, which gives an impression of callosal thickening at first glance, may be an imaging clue to detect female patients with CASK mutations.
Acknowledgments
We thank Dr Shinichiro Hamano at Saitama Children's Medical Center for referring a patient, and the patients and families for their contribution to this study.
Footnotes
-
Drs Takanashi and Arai are funded by the research grant (20A-14) for Nervous and Mental Disorders from the Ministry of Health, Labour and Welfare of Japan. Drs Hayashi and Inazawa are funded by Health and Labor Sciences Research Grants from the Ministry of Health, Labour and Welfare, Japan; and a grant from the New Energy and Industrial Technology Development Organization. Dr Okamoto was funded by Health and Labor Research Grants in 2009 by the Ministry of Health, Labour and Welfare in Japan. Dr Barkovich is funded by the National Institutes of Health (multiple research grants).
Indicates open access to non-subscribers at www.ajnr.org
References
- Received February 24, 2010.
- Accepted after revision April 5, 2010.
- Copyright © American Society of Neuroradiology