Abstract
BACKGROUND AND PURPOSE: MR imaging and 1H-MR spectroscopy changes are well reported in cirrhotic patients, whereas they are inadequately reported in EHPVO. The aim of this study was to investigate age-related changes in brain MR imaging and metabolite profile in EHPVO with and without MHE and to explore any correlation of imaging and 1H-MR spectroscopy parameters with blood ammonia.
MATERIALS AND METHODS: Sixty-three patients with EHPVO (children, 7–12 years [n = 22], adolescents, 13–18 years [n = 15] and adults, 19–41 years [n = 26]) and 47 healthy age/sex-matched volunteers were studied. Neuropsychological tests, MR imaging, 1H-MR spectroscopy, and blood ammonia estimation were performed in all subjects.
RESULTS: Of 63 EHPVO patients, 25 (40%) who had MHE showed significantly increased MD, Glx, and blood ammonia in all 3 age groups; however, myo-inositol was significantly lower only in adults when compared with controls. MD positively correlated with blood ammonia and Glx in all age groups. Brain choline levels were normal in all patients with different age groups.
CONCLUSIONS: Increases in brain MD, Glx, and blood ammonia were associated with MHE in all age groups. Normal brain choline in EHPVO signifies healthy liver and may serve as a diagnostic marker for its differentiation from cirrhosis-induced encephalopathy. Significant decrease of myo-inositol in adults is probably due to cellular osmoregulation secondary to long-standing hyperammonemia.
Abbreviations
- ALIC
- anterior limb of internal capsule
- ALT
- alanine transaminase
- ANOVA
- analysis of variance
- AST
- aspartate transaminase
- BA
- blood ammonia
- CG
- cingulate gyrus
- CN
- caudate nucleus
- DTI
- diffusion tensor imaging
- EHPVO
- extrahepatic portal vein obstruction
- FA
- fractional anisotropy
- FWM
- frontal white matter
- Glx
- glutamine/glutamate
- GP
- globus pallidus
- 1H-MRS
- 1H-MR spectroscopy
- MD
- mean diffusivity
- MHE
- minimal hepatic encephalopathy
- NAA
- N-acetylaspartate
- NS
- not significant
- OWM
- occipital white matter
- P
- putamen
- PLIC
- posterior limb of internal capsule
- PT
- prothrombin time
- S
- significant
- SB
- serum bilirubin
- Sp
- splenium
- T
- thalamus
Extrahepatic portal vein obstruction is a condition in which there is mechanical obstruction to portal venous flow. It may be a partial or complete obstruction of the main portal vein with or without obstruction to its tributaries, in the absence of liver cirrhosis or malignancy.1 It is an important cause of noncirrhotic portal hypertension, especially in developing countries.2 The quality of life in patients with EHPVO is superior to those with liver cirrhosis, because hepatocellular function is normal. Furthermore, the condition is thought to be nonprogressive, and effective therapy is available for variceal bleeding, which is the major source of morbidity in EHPVO.3 Recent studies have reported development of MHE in EHPVO.3,4 Hepatic encephalopathy occurring in conditions in which portal blood bypasses the liver has been classified as type B hepatic encephalopathy.5
Abnormal cognitive functions interfere significantly with the daily functioning and lead to impairment of health-related quality of life.6–8 It is generally agreed that hepatic encephalopathy occurs due to the effect of toxins on the brain, with the most important being ammonia.9 Ammonia undergoes a high degree of extraction in the liver, and thus portosystemic shunt surgery induces a rise in plasma ammonia and results in the development of hepatic encephalopathy in patients with cirrhosis, especially after transjugular intrahepatic portosystemic shunt surgery or surgical shunts.4 Hyperammonemia and hepatic encephalopathy secondary to portosystemic shunt surgery, despite normal hepatocellular functions, have been demonstrated in patients with congenital portosystemic shunt surgery.10
Brain MR imaging, 1H-MR spectroscopy, diffusion and DTI, magnetization transfer, and functional MR imaging have been used to understand the pathophysiological alterations in patients with cirrhosis-induced hepatic encephalopathy.11,12 A classical triad of increased resonance of Glx along with decreased myo-inositol and choline are considered to be the hallmark of hepatic dysfunction with hepatic encephalopathy in patients with liver cirrhosis on 1H-MR spectroscopy.12–14 However, in patients with EHPVO, such information is sparse.4 Reduced myo-inositol and increased Glx along with normal choline has been demonstrated in adult EHPVO subjects.4 Nevertheless, isolated changes in Glx have been reported in children with EHPVO having abnormal cognitive functions.15
The aim of this study was to investigate age-related changes in brain MR imaging and metabolite profile in EHPVO with and without MHE and to explore any correlation of imaging metrics and 1H-MR spectroscopy parameters with blood ammonia.
Materials and Methods
Sixty-three stable EHPVO patients (22 children, 15 adolescents, and 26 adults) were studied. The age group for children was 7–12 years, for adolescents, 13–18 years, and for adults, 19–41 years. Forty-seven healthy age/sex-matched controls were also included and classified into 3 age groups: 7–12 years (n = 17), 13–18 years (n = 8), and 19–41 years (n = 22). All subjects underwent a battery of neuropsychological tests, clinical examinations, blood ammonia estimation, MR imaging, and 1H-MR spectroscopy.
Neuropsychological Tests
Each subject completed a neuropsychological test battery especially designed for children, adolescents, and adults, which was adopted for an Indian population to identify abnormalities in neuropsychological functions. The Revised Amsterdamse Kinder Intelligentie Test containing a battery of 9 subsets was used for children.16 However, trail-making tests and a performance subset of the modified Wechsler Adult Intelligence Scale was used for adolescents and adults. The patients were considered to have MHE if they had 2 or more abnormal neuropsychological tests,11,17 while the rest of the patients with less than 2 abnormal neuropsychological tests were considered to have no MHE.
Diagnosis of EHPVO
Diagnosis of EHPVO was based on consensus guidelines of the Asian Pacific Association for the Study of Liver.1 EHPVO patients included in this study either presented with splenomegaly or variceal bleeding or both. We could not find any evidence of procoagulant disorders or any specific disease predisposing to portal vein thrombosis in these patients and they were presumed to be “idiopathic”. The presumed duration of illness in the pediatric age group was 5.5 ± 3.4 years, in adolescents it was 6.2 ± 4.6 years, and in adult patients it was 8.15 ± 5.4 years. This quantification of duration was based on the first clinical symptoms reported by the patients such as variceal bleeding or splenomegaly or both. EHPVO patients with a history of overt hepatic encephalopathy in the past, recent upper gastrointestinal bleeding, antibiotic intake in the last 6 weeks, evidence of chronic liver disease on investigation, or any neurologic/psychiatric disorders were excluded from the study. The study protocol was approved by our institutional ethics committee, and written informed consent was obtained from each subject or from caregivers. All patients were evaluated with blood tests (hemogram, liver function tests, and prothrombin time), imaging (abdominal sonography, Doppler for portal vein), and upper gastrointestinal endoscopy for assessment of varices. Spleen size was determined by clinical examination. The spleen was considered to be enlarged when it was palpable below the costal margin, and its maximal length from the 11th rib was measured by using a tape. In controls the spleen was not palpable and its length was considered to be 0. Blood ammonia was determined before MR imaging and after overnight fasting by using a standard enzymatic ultraviolet method.
MR Imaging and 1H-MR Spectroscopy
MR imaging was performed on a 1.5T MR imaging scanner (Signa LX, 9.1; GE Healthcare Technologies, Milwaukee, Wisconsin) by using a standard quadrature birdcage transmit-and-receive radio frequency head coil. T1-weighted, T2-weighted, and diffusion tensor images were performed in all patients and age/sex-matched controls. T1-weighted images were used for placement of spectroscopy voxel; however, T2-weighted imaging was used to look for any structural abnormality. T1-weighted fast spin-echo imaging with TR of 1000 ms, TE of 14 ms, NEX of 2, and T2-weighted fast spin-echo imaging using TR of 6000 ms, TE of 85 ms, and NEX of 4 were taken. DTI was acquired by using a single-shot echo-planar dual SE sequence with ramp sampling.18 A balanced rotationally invariant19 diffusion encoding scheme with 10 uniformly distributed directions over the unit sphere was used for obtaining the diffusion-weighted data. The b factor was set to 0 and 1000 s/mm2, TR of 8 s, and TE of 100 ms. A total of 36 axial sections were acquired with image matrix of 256 × 256 (following zero-filling) section thickness of 3 mm with no intersection gap and a FOV of 240 × 240 mm2 in T1-weighted, T2-weighted, and diffusion tensor images. To enhance the signal intensity–to-noise ratio and reduce the phase fluctuations, magnitude-constructed images were repeated (NEX of 8) and temporally averaged.
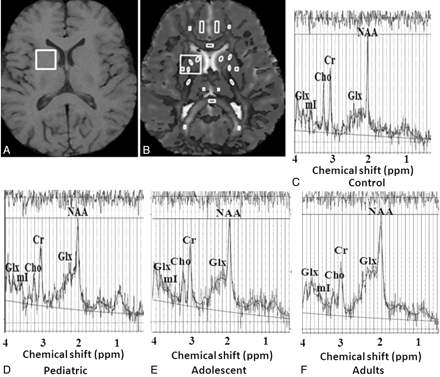
Spectra were obtained with and without water suppression. Localized single voxel point-resolved spectroscopy included TR/TE of 3000 ms/35 ms and number of averages of 64. A voxel of 2 × 2 × 2 cm3 was located mainly in the right basal ganglion region of the brain in all cases. It has been reported that changes in basal ganglia are most characteristic of hepatic encephalopathy in cirrhosis with MHE20,21; hence, this specific location was selected for spectroscopy voxel placement. The voxel localization was guided by the T1-weighted axial images as shown in Fig 1A. After global shimming, voxel shimming was performed, and a full width at half maximum of 4–6 Hz was achieved in all cases. The total time required for MR imaging and 1H-MR spectroscopy was 17 minutes 30 seconds.
A, Shows spectroscopy voxel placed on right basal ganglia region in healthy adult control on T1-weighted image. B, Axial gray scale fractional anisotropy map fused with mean diffusivity map shows regions of interest placement at the level of third ventricle from where FA and MD values have been obtained. In addition, large spectroscopy voxel co-registered with this map shown in this figure to quantify FA and MD values for the purpose of correlation of these indices (Fig 2). C–F, LC Model processed localized 1H-MR spectroscopy from the right basal ganglia region show metabolite pattern of choline, Glx, myo-inositol, and NAA in adult control (C), EHPVO child (D), EHPVO adolescent (E), and EHPVO adult (F). The EHPVO child and adolescent spectra show increased Glx while EHPVO adult shows increased Glx and decreased myo-inositol compared to control. Values of choline, creatine, and NAA in all age groups of patients is comparable to controls (Table 4).
Data Analysis and Quantification
The DTI data were processed as described in detail elsewhere.22 Briefly, after image cropping and distortion corrections, the data were interpolated to attain isotropic voxels and decoded to obtain the tensor field for each voxel. The tensor field data were then diagonalized by using the analytic diagonalization method22 to obtain the eigenvalues (λ1, λ2, and λ3) and the 3 orthonormal eigenvectors. The tensor field data and eigenvalues were used to compute the DTI metrics such as MD and FA for each voxel.
Regions of interest were placed at the level of third ventricle on bilateral internal capsules (posterior and anterior limb), bilateral caudate nuclei, putamen, thalami, globus pallidus, cingulate gyrus, frontal and occipital white matter, genu, and splenium in patients and healthy controls to quantify MD and FA (Fig 1 B). We selected these regions of interest, as these structures have been shown to be abnormal in previous studies11,12 and they may be affected in hepatic encephalopathy. The size of the regions of interest varied from 3.5 to 32 mm2, with shape varying from elliptical to rectangular. Additionally, coordinates of spectroscopy were co-registered with MD maps in each subject, and MD and FA values from this large voxel (right basal ganglion region) were also measured to correlate these with spectroscopy-derived metabolites and blood ammonia (Fig 1B).
For processing and quantification of all individual 1H-MR spectroscopy data (Fig 1 C–F), the LCModel software package (LCModel 6.2; Stephen Provencher, Oakville, Ontario, Canada) was used.23 The metabolite concentrations of NAA, choline, creatine, Glx, and myo-inositol were calculated. After processing with the LCModel, spectral data with line width at full width at half maximum of >6 Hz and signal intensity–to-noise ratio of >3 were included in the study. All spectra were assessed for general quality by experienced observers.
Statistical Analysis
Left and right measurements of DTI metrics of all regions except for genu and splenium were pooled together, and averaged values of different regions were used for the final dataset for statistical analysis. ANOVA post hoc Bonferroni multiple comparisons were performed to detect the difference in EHPVO with and without MHE as compared with controls in the biochemical parameters, blood ammonia, DTI metrics, and metabolites. To study the relationship between 1H-MR spectroscopy coordinate-derived MD with blood ammonia and metabolites, the Pearson correlation coefficient was computed. For computation of Pearson correlation, the values of different parameters in all 3 age groups were pooled together. A value of P < .05 was chosen to establish significance between the groups. All statistical data computations were performed by using SPSS (version 16.0, SPSS, Chicago, Illinois).
Results
MHE was present in 7 of 22 children (32%), in 6 of 15 adolescents (40%), and in 12 of 26 (46%) adults. The frequency of MHE in patients with EHPVO increased with age. The clinical and biochemical parameters of patients with and without MHE in all 3 age groups did not show any significant difference as compared with controls except that blood ammonia and spleen size were significantly higher in all age groups of patients compared with controls (on-line Table 1). In this study, 71% of patients presented with acute variceal bleed and splenomegaly, whereas 29% of patients presented with only asymptomatic splenomegaly.
Quantitation of DTI Metrics
A significantly increased MD was observed in 8 of 11 brain regions in the pediatric age group with MHE, whereas it was significantly increased in 9 of 11 brain regions of adolescent and adult groups having MHE. In the thalamus region, MD did not show any significant increase in pediatric MHE patients (on-line Table 2). No significant change was observed in the FA value in any brain region in patients having MHE and no MHE as compared with controls in all age groups (on-line Table 3).
1H-MR Spectroscopy
An increasing trend of Glx was observed from no MHE to MHE in all 3 age groups compared with controls (on-line Table 4). A significant increase in Glx was found in patients with MHE as compared with controls in all age groups.
Among children, the myo-inositol did not show any significant differences between controls and the MHE group (on-line Table 4). Among adolescents, although myo-inositol was decreased in patients with MHE compared with controls, this was not statistically significant (on-line Table 4). In adults, myo-inositol was significantly decreased in patients with MHE compared with controls (on-line Table 4). Thus, significant myo-inositol depletion was identified only in adult patients with MHE. In all 3 age groups, no statistically significant difference was observed in the other metabolites (eg, choline, creatine, and NAA) between controls and patients with and without MHE (on-line Table 4).
Correlations across DTI Metrics, Glx, and Blood Ammonia
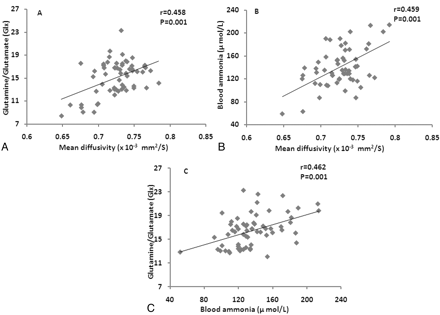
A significant positive correlation of MD with Glx and blood ammonia was observed (Fig 2A, -B). MD was obtained from a spectroscopy voxel co-registered to a MD map in all age groups. No correlation was observed between FA with Glx and blood ammonia. A significant positive correlation was observed between Glx and blood ammonia (Fig 2C).
Scatterplots show significant positive correlation of mean diffusivity values derived from coordinates of spectroscopy (Fig 1 B) with Glx (A) and blood ammonia (B). Scatterplot (C) shows significant positive correlation between blood ammonia and Glx in these patients. All age group data of extrahepatic portal vein obstruction patients were pooled together for the estimation of correlation.
Discussion
It is generally accepted that increased blood ammonia induces brain glutamine synthesis in the astrocytes, which results in cognitive impairment in cirrhotic patients.24,25 Minguez et al4 have reported increased blood ammonia in adult EHPVO patients with minimal encephalopathy. In the present study, an increasing trend of blood ammonia levels was observed from no MHE to MHE in 1ll 3 age groups compared to controls. This may be due to increased extrahepatic collateral vessels, resulting in increased shunt surgery of ammonia directly into the blood from the gut, resulting in further impairment in cognitive functions in these patients with advancing age.
Cerebral edema is a well-known feature of acute liver failure; however, recently it has also been reported in patients with chronic liver disease.9,11 In this study, increased MD in both gray and white matter with no significant change in FA indicate generalized low-grade cerebral edema in patients with MHE without any microstructural changes in all age groups as compared with controls. It appears that the increase in interstitial brain water was not sufficient enough to cause separation of white matter fibers in MHE patients, though it was clearly quantifiable on MD maps. Positive correlation of MD with blood ammonia and Glx indicates that ammonia may be responsible for low-grade cerebral edema in patients with EHPVO having MHE in all age groups of patients.
1H-MR spectroscopy is a noninvasive technique that has been used in the assessment of cerebral metabolite changes in cirrhosis-induced hepatic encephalopathy. Increased Glx has been shown to be associated with increased glutamine concentration in the brain, a finding that has been attributed to increased detoxification of ammonia by astrocytes to glutamine via the amidation of glutamate.26 In the present study, increased Glx in EHPVO patients having normal liver functions in all 3 age groups appears to be due to portosystemic shunt surgery, resulting in increased blood ammonia. The extent of portosystemic shunt surgery appears to be a key factor in the development of MHE in the absence of intrinsic hepatocellular disease in patients with EHPVO. A significant positive correlation between blood ammonia and Glx heightens our suspicion that ammonia is in some way responsible for increased brain Glx and lends support to the hypothesis that portosystemic shunt surgery exposes the brain to ammonia in patients with EHPVO. In the present study, no significant change in choline was observed in EHPVO patients of all age groups and is similar to the findings reported by Minguez et al.4 A lack of significant difference in choline in EHPVO patients compared with controls can be explained on the basis of normal de novo synthesis of choline by the normal functioning liver. No change in choline in patients with EHPVO having MHE indicates that choline alteration on 1H-MR spectroscopy is related to liver dysfunction and does not influence the development of MHE. Based on the data from this study, it appears that the brain choline alteration is secondary to liver dysfunction and may not be a participant in the pathogenesis of MHE. This may serve as a diagnostic marker for its differentiation from cirrhosis-induced MHE.
Myo-inositol is located in astrocytes and is the most important organic osmolyte or cell volume regulator.27 It has been demonstrated that an increase in intracellular osmolality secondary to accumulation of glutamine is compensated for by a decrease in intracellular myo-inositol and other osmolytes.28 In patients with cirrhosis with or without hepatic encephalopathy, a low level of myo-inositol has been reported compared with controls.13,21 A similar reduction in myoinositol has been reported in adult EHPVO subjects.4 However, in acute liver failure patients, no significant change in myo-inositol has been observed, indicating that there is insufficient time for osmolytes to compensate for significantly increased glutamine.29 In the present study, no significant change in myo-inositol in children and adolescents having MHE compared with controls suggests that hyperammonemia exposure to the brain appears to be of insufficient duration, which probably does not disturb the astrocyte volume homeostasis. This manifests as age progresses in adults, resulting in decreased myo-inositol that is associated with the pathogenesis of MHE in adult EHPVO patients. However, lack of myo-inositol reduction in pediatric and adolescent groups suggests that caution may be exercised while interpreting the 1H-MR spectroscopy data in these groups of EHPVO patients. Foerster et al30 have reported 1H-MR spectroscopy changes in cirrhotic children with MHE similar to those of adult cirrhotics with MHE. This difference in metabolite concentration in MHE of EHPVO children and MHE of cirrhotic children appears to be due to the difference in liver function in these 2 groups.
A classical triad of increased Glx with decreased myo-inositol and choline in children as well as adults with cirrhosis with or without MHE has been reported on brain 1H-MR spectroscopy and is considered to be helpful in establishing a diagnosis of MHE in these patients.12–14,30 In patients of adult EHPVO with MHE, increased Glx and decreased myo-inositol along with no change in choline has been reported on brain 1H-MR spectroscopy.4 These observations have been confirmed in a larger number of adult EHPVO patients in this study, suggesting that the presence of normal choline along with increased Glx may be used as signature of EHPVO-related encephalopathy in all age groups. In this study we observed no significant changes in metabolite concentrations on 1H-MR spectroscopy in the no-MHE groups, whereas on DTI, few regions showed significant changes in MD in no-MHE groups as compared with controls, suggesting that it may be possible to separate MHE from no MHE in patients with EHPVO at all ages.
It is presently thought that MHE should be treated, as it is known to affect the health-related quality of life in these patients.31 Neuropsychological tests are the only methods used in its diagnosis and management. However, these tests are associated with subjectivity of the individuals involved in their assessment. Significantly increased brain water as measured by MD, as well as an increase in brain Glx, an indirect marker of brain ammonia in these EHPVO patients with MHE compared with controls and no-MHE patients, suggests that these may be used as alternative objective methods for the diagnosis and management of MHE.
Conclusions
Increases in MD, Glx, and blood ammonia are associated with MHE in all age groups. No change of choline in EHPVO may serve as a diagnostic marker for its differentiation from cirrhosis-induced MHE. A significant decrease of myo-inositol only in adult EHPVO patients suggests that it takes a long time to compensate for the rise in intracellular osmolality caused by Glx. However, a lack of myo-inositol reduction in pediatric and adolescent groups suggests that caution may be exercised while interpreting 1H-MR spectroscopy data in these groups of EHPVO patients.
Footnotes
Santosh K. Yadav acknowledges financial assistance from the Indian Council of Medical Research, New Delhi, India.
-
Indicates article with supplemental on-line table.
Indicates open access to non-subscribers at www.ajnr.org
References
- Received November 24, 2009.
- Accepted after revision December 31, 2009.
- Copyright © American Society of Neuroradiology