Abstract
BACKGROUND AND PURPOSE: The larynx is a rare site for primary NHL. Fewer than 100 cases have been reported in the literature, with the largest imaging review involving only 4 patients. We describe the findings of laryngeal lymphoma on CT, PET, and MR imaging and identify features that may distinguish laryngeal lymphoma from the far more common laryngeal squamous cell carcinoma.
MATERIALS AND METHODS: Multi-institutional retrospective chart review revealed 20 patients with histopathologically proved laryngeal lymphoma. Pretreatment CT, PET, and MR images were reviewed by a head and neck radiologist, focusing on extent of tumor, cervical lymph node involvement, and enhancement patterns.
RESULTS: Patients ranged from 30 to 90 years of age with a mean of 63 years at the time of initial diagnosis and a 2:1 female predominance. The average tumor size was 37 ± 19 mm. In all patients, laryngeal lymphoma involved the supraglottis but also extended into the glottis (65%) and hypopharynx (60%). The subglottis was less frequently involved (35%). Laryngeal cartilage invasion and cervical lymphadenopathy were each seen in 20% of patients. Lymphoma was consistently FDG-avid (100%) and usually enhanced uniformly with iodinated contrast (73%). Necrosis and calcification were not seen in any cases.
CONCLUSIONS: Although laryngeal lymphoma is rare, particular imaging features suggest this diagnosis. A large uniformly enhancing supraglottic tumor without central necrosis and without cervical lymphadenopathy is a characteristic finding of lymphoma. Similar to squamous cell carcinoma, lymphoma may extend into the subglottis, pharynx, and laryngeal cartilages.
Abbreviations
- FDG
- fluorodeoxyglucose
- MALT
- mucosa associated lymphatic tissue
- NHL
- non-Hodgkin lymphoma
- PET
- positron-emission tomography
Primary NHL of the larynx is a rare entity accounting for <1% of laryngeal tumors; however, it is the second most common primary hematologic tumor of the larynx after plasmacytoma.1–3 Fewer than 100 cases have been reported in the literature, with the largest review of imaging involving 4 patients.4 Although 90% of tumors involving the larynx are squamous cell carcinoma,5 laryngeal lymphoma is an important differential consideration because lymphoma is treated with chemoradiation instead of surgery.1–2,6–9 Early detection of lymphoma allows improved patient care and may result in decreased dissemination of the disease process.10,11
The purpose of this study was to identify imaging features of laryngeal lymphoma that could differentiate it from the far more common squamous cell carcinoma of the larynx and thus aid in the early detection of this rare tumor and lead to its proper treatment.
Materials and Methods
A multi-institutional retrospective chart review revealed 20 patients with histopathologically proved laryngeal lymphoma. Only pretherapeutic cross-sectional imaging studies were included in the analysis. The study extended from July 2002 to December 2008 and involved multiple different imaging protocols and hardware. CT included enhanced and unenhanced axial 3- to 5-mm-collimation images in both soft-tissue and bone algorithms. MR imaging was performed on 1.5T systems and consistently included pre- and postcontrast T1-weighted spin-echo sequences and T2-weighted spin-echo imaging in multiple planes. PET-CT was performed with intravenous fluorine [18F] FDG with a dosage range of 10–20 mCi.
Clinical records were reviewed for patient demographics and histopathologic lesion characteristics. The MR imaging, CT, and PET images were assessed for site of tumor origin, tumor extent, and nodal disease. The metabolic activity, signal intensity, radioattenuation, and enhancement patterns of the tumors were also evaluated. Review of the images was performed by a dedicated head and neck radiologist with 7 years in practice.
Results
Patients' ages ranged from 30 to 90 years, with a mean age of 63 years at the time of diagnosis. A 2:1 female predominance was noted, with 13 women and 7 men. According to the World Health Organization classification for lymphoma,12 85% (17/20) of cases were B-cell lymphoma and 15% (3/20) of cases were T- and NK-cell lymphoma. All 3 T- and NK-cell lymphoma cases were CD56-positive, 1 of which was weakly positive, and thus were classified as NK/T-cell phenotypes. The B-cell lymphomas were further classified into diffuse large B-cell lymphomas, which were seen in 50% (10/20); MALT, which was seen in 20% (4/20); follicular type, in 10% (2/20); and mantle cell, in 5% (1/20) of the subjects. In all cases, the larynx was considered to be the primary site of tumor, on the basis of radiologic, clinical, and histopathologic data.
Fourteen contrast-enhanced CT, 4 PET-CT, and 2 MR imaging studies were reviewed. Lymphoma of the larynx always involved the supraglottic region (20/20) (Fig 1A, –B), with contiguous involvement of the glottic region in 65% (13/20) and subglottic region in 35% (7/20). There were no cases in which lymphoma was isolated to the subglottis or glottis. Histopathologically, the lymphoma was submucosal in 47% (7/15) (Fig 2) and mucosal in 13% (2/15); both the submucosal and mucosal regions were involved in 40% (6/15). In the remaining 5 patients, the distinction between mucosal and submucosal location was not specified on pathology reports. The tumor was seen on the left side in 35% (7/20) and on the right side in 40% (8/20) and was bilateral in 25% (5/20).
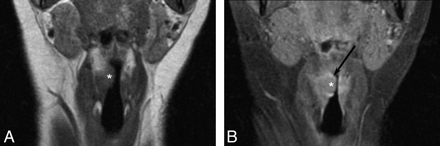
A 65-year-old woman with laryngeal lymphoma. A, Coronal T1-weighted MR image demonstrates an isointense supraglottic soft-tissue nodule within the right false vocal cord (asterisk). B, Coronal T1-weighted contrast-enhanced MR image shows enhancement of the nodular lesion (asterisk) and the adjacent mucosa of the supraglottic larynx (arrow), which was also found to be positive for lymphoma.
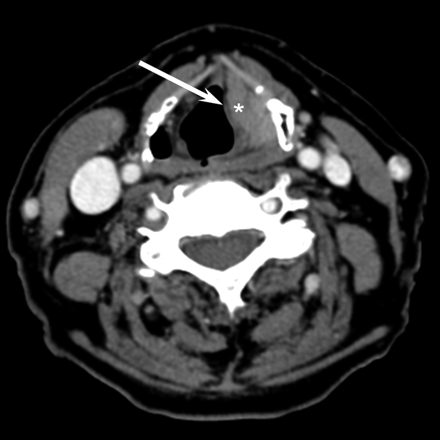
A 65-year-old woman with laryngeal lymphoma. Axial CT scan demonstrates a uniformly enhancing mass involving the left false vocal cord (asterisk). Note the thin layer of fat separating this submucosal lesion from the mucosa (arrow).
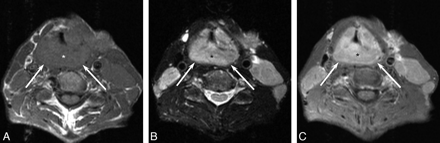
Intralaryngeal tumor involved the false cords in 85% (17/20), aryepiglottic folds in 65% (13/20), true cords in 65% (13/20), epiglottis in 55% (11/20), and laryngeal cartilage in 20% (4/20) (Fig 3). The lymphoma extended beyond the larynx into the hypopharynx in 60% (12/20) (Fig 4), the strap muscles in 20% (4/20), the tongue base in 10% (2/20), and the oropharynx in 25% (5/20). Cervical lymphadenopathy was noted in 20% (4/20) of the cases.
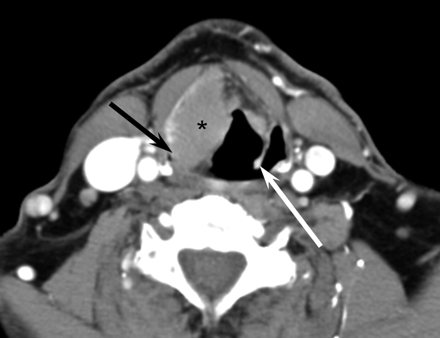
A 53-year-old man with laryngeal lymphoma. Axial CT scan demonstrates moderate uniform enhancement of a right false vocal cord mass (asterisk) with involvement of both the right aryepiglottic fold and the right pyriform sinus (black arrow). Note the normal configuration of the left aryepiglottic fold (white arrow), which is indistinguishable on the right.
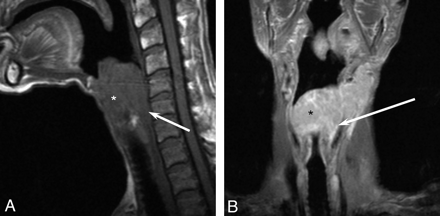
A 64-year-old man with laryngeal lymphoma. A, Sagittal T1-weighted MR image demonstrates a large isointense supraglottic laryngeal tumor (asterisk ) with posterior extension to the hypopharynx (arrow ). B, Coronal T1-weighted contrast-enhanced MR image shows homogeneous enhancement of the large supraglottic lesion (asterisk ) with posterolateral extension into the hypopharynx (arrow ).
Laryngeal lymphoma was consistently metabolically active, demonstrating FDG avidity in all 4 cases with PET-CT imaging (Fig 5). The tumors showed uniform contrast enhancement in 73% (8/11) and heterogeneous enhancement in 18% (2/11). No necrosis or calcifications were seen on CT imaging. MR imaging demonstrated intermediate T1-weighted and variable T2-weighted signal-intensity characteristics. Homogeneous enhancement was evident with contrast administration (Fig 6).
A 65-year-old woman with laryngeal lymphoma. Axial PET-CT image demonstrates avid FDG uptake in a focal right false vocal cord lesion.
A 64-year-old man with laryngeal lymphoma (same patient as in Fig 4). A, Axial T1-weighted MR image demonstrates an isointense large supraglottic mass (asterisk ) extending into the hypopharynx (arrows ). B, Axial T2-weighted MR image shows the mass to be hyperintense (asterisk ). It is again seen extending into the hypopharynx (arrows ). C, Axial T1-weighted contrast-enhanced MR image demonstrates homogeneous enhancement (asterisk ) of the mass, which is again seen to be extending into the hypopharynx (arrows). Note the bulky bilateral lymphadenopathy.
Discussion
NHL most frequently arises in the gastrointestinal tract, but the head and neck is the second most frequent region.13 Most head and neck lymphoma arises within the Waldeyer ring, but extranodal extralymphatic sites still accounted for 25% of these tumors. The most common extranodal extralymphatic sites were the paranasal sinuses, the salivary glands, and the thyroid gland.14 The larynx is, thus, a rare site for a primary NHL.
NHL is presumed to arise from 2 locations within the larynx: 1) aggregates of specialized lymphoid tissue in the submucosa, predominantly of B-cell lineage, or 2) MALT, predominantly arising from the aryepiglottic folds and epiglottis.15 Fewer than 100 cases of lymphoproliferative tumors arising from the larynx (including both NHL and immunosuppression-related lymphoproliferative diseases) have been previously reported in the literature.4
The symptoms of NHL are typically site-specific and thus have manifestations similar to those produced by epithelial carcinomas in the same location; these typically include dysphonia, hoarseness, dysphagia, and cervical lymphadenopathy.16 The macroscopic appearance of laryngeal lymphoma is described as a smooth submucosal swelling or polypoid mass without ulceration, which may be a useful feature for endoscopic diagnosis.17 Substantial tissue quantity is necessary for specific histologic characterization. Immunohistochemistry can determine B-cell lineage, which demonstrates CD19, CD20, and CD22 immunoglobulins, or T-cell lineage, which demonstrates CD2 and CD3.18 NK-cell lymphoma, a rare subclassification of T-cell lymphoma with a poor prognosis, expresses the cell adhesion molecule CD56 and demonstrates an aggressive angiocentric growth pattern.19 Furthermore, cytogenetic and molecular studies can be performed to tailor the therapeutic approach.18 Because NHL is considered highly radiosensitive, the literature is supportive of radiation therapy as the treatment of choice for primary lesions with or without regional lymph node involvement.2,12 However, in patients with more disseminated disease, a combination of radiation and chemotherapy is favored.13,20
Previous reports have suggested a male predominance of laryngeal lymphoma6,14; however, our study indicated a 2:1 female predominance, in keeping with the study of King et al.4 Additionally, two-thirds of reported primary laryngeal lymphoma was left-sided, with most tumors arising in the supraglottis, specifically the epiglottis and aryepiglottic folds.5,21 Our study, however, identified no predilection for either side. It is likely that predispositions to one side or one sex are the result of statistical clustering in a particular series.
In our series, pretreatment imaging demonstrated that laryngeal lymphoma always involved the supraglottis and occasionally extended into the glottis and less frequently the subglottis. The tumor most frequently involved the false cords and aryepiglottic folds but was also frequently seen in the epiglottis and true cords. These findings are compatible with prior endoscopic reports, which documented a predilection for the epiglottis, aryepiglottic folds, and vestibule.5,22,23
On pathologic evaluation, 90% of reported cases demonstrated a submucosal component, with only 10% being primarily mucosal in origin. Most interesting, the localized mucosal cases in our series were 2 of the 3 NK/T-cell lymphoma phenotypes, with the third NK/T-cell lymphoma phenotype demonstrating involvement of both the mucosa and submucosa. This supports a preference of NK/T cell lymphoma for the mucosa.15 Furthermore, the supraglottic and primarily submucosal location of laryngeal lymphoma is in keeping with prior radiographic imaging reported by Takayama et al24 and King et al.4
Most reported cases of laryngeal lymphoma have been of the B-cell phenotype, with only a small percentage being of the NK/T-cell phenotype.5,21,24 Our study was consistent with this finding, demonstrating a B-cell phenotype to NK/T-cell phenotype ratio of 6:1.25 We found that the diffuse large B-cell lymphoma was the most common immunophenotype, consistent with prior reports,25 followed by the MALT. As previously noted, the major imaging and pathologic distinction between the B-cell phenotype and the NK/T-cell phenotype was the involvement of the submucosa in all the cases with B-cell phenotype. Our findings suggest that the B-cell phenotype primarily affects the submucosa and secondarily involves the mucosa. This involvement is likely due to the aggregates of B-cell-specific lymphoid tissue within the submucosa of the larynx.15
In our series, most cases of laryngeal lymphoma demonstrated uniform moderate enhancement with intravenous contrast. The 2 cases that demonstrated heterogeneous enhancement were of the B-cell phenotype and did not otherwise illustrate a distinctive immunophenotype or other distinguishing feature. Lack of enhancement may also be seen in posttherapy CT, likely indicating treatment response.26 There was no CT evidence of marked central necrosis or abnormal calcifications, features that might be useful for suggesting submucosal laryngeal lesions such as chondrosarcoma or other more aggressive neoplasms. Extralaryngeal tumor extension was most frequently noted to involve the hypopharynx and, less commonly, the oropharynx and strap muscles. However, 25% of cases were seen involving only the supraglottic larynx, indicating that laryngeal lymphoma does not always extend beyond the supraglottis as previously suggested.21 The extent of local spread is most likely related to the onset of symptoms and the time of imaging. However, the involvement of the strap muscles, tongue base, and oropharynx is of little diagnostic utility, because it is commonly seen with squamous cell carcinoma and is thus not a distinguishing feature. Cervical lymphadenopathy predominately involved the internal jugular chain and was seen in 20% of our cases in accordance with the previously reported value of 25%.5 This finding may be of diagnostic utility because cervical adenopathy is commonly seen in supraglottic squamous cell carcinoma. Lymphoma of the larynx, similar to lymphoma in other parts of the body, is metabolically active on PET-CT, a finding that may be of further utility in the future as molecular imaging continues to evolve.27
MR imaging of laryngeal lymphoma demonstrated homogeneous intermediate T1-weighted and variable T2-weighted signal intensity and homogeneous gadolinium enhancement. The homogeneous signal-intensity characteristics of our lesions are consistent with the MR imaging of Fujita et al28 and Takayama et al24; however, heterogeneous intensity on T2-weighted and contrast-enhanced images has been reported by King et al.4 The variability in T2 signal intensity, as previously suggested, may represent the underlying cellularity of the tumor28 and thus is likely to differ from lesion to lesion. However, MR signal-intensity characteristics are still diagnostically nonspecific, so the technique is primarily used for determination of the extent of tumor involvement.28 Future use of diffusion-weighted imaging may improve specificity because lymphomas elsewhere show restricted diffusion compared with many other neoplasms.29 Diffusion-weighted imaging was not performed in the cases included in our series.
In addition to squamous cell carcinoma and laryngeal lymphoma, there are other lesions that belong in the differential diagnosis of a uniformly enhancing mucosal or submucosal laryngeal mass. Paraganglioma, schwannoma, venolymphatic malformation, adenoid cystic carcinoma and other minor salivary tumors, rhabdomyosarcoma, chondrosarcoma, leiomyoma, spindle cell neoplasms, angiosarcoma, fibrosarcoma, enchondroma, tuberculoma, tracheobronchial papilloma, and rheumatoid arthritis should all be considered.
Conclusions
Although laryngeal lymphoma is much less common than laryngeal squamous cell carcinoma, there are certain imaging characteristics that should suggest lymphoma. A large non-necrotic supraglottic lesion with a submucosal component that demonstrates homogeneous enhancement is more characteristic of lymphoma than of squamous cell carcinoma. Laryngeal lymphoma is commonly seen to extend into the hypopharynx, but it can also be isolated to the supraglottis. Calcifications are not seen with laryngeal lymphoma. Like squamous cell carcinoma, lymphoma can extend into the subglottis, oropharynx, strap muscles, and laryngeal cartilage. Cervical lymphadenopathy, a common finding in supraglottic squamous cell carcinoma, is seen in a smaller percentage of patients with laryngeal lymphoma.
Footnotes
-

-
Paper previously presented at: 47th Annual Meeting of the American Society of Neuroradiology, May 16–21, 2009; Vancouver, British Columbia, Canada.
References
- Received November 9, 2009.
- Accepted after revision January 26, 2010.
- Copyright © American Society of Neuroradiology