Abstract
SUMMARY: Reversible encephalopathy after transplantation is well recognized. The condition is commonly thought to be related to immune suppression, and a characteristic brain imaging pattern is typically recognized with vasogenic edema in the parietal and occipital regions, typically termed posterior reversible encephalopathy syndrome (PRES). We report the case of a patient with reversible encephalopathy after cardiac transplantation with brain biopsy evidence of endothelial activation, selective intravascular/perivascular T-cell trafficking, and VEGF expression in astrocytes, neurons, and the endothelium.
Reversible encephalopathy after transplantation is well recognized. A characteristic brain-imaging pattern is typically seen with vasogenic edema in the parietal and occipital regions, typically termed posterior reversible encephalopathy syndrome (PRES). We report the case of a patient with reversible encephalopathy after cardiac transplantation with brain biopsy evidence of endothelial activation, selective intravascular/perivascular T-cell trafficking, and endothelial/cellular vascular endothelial growth factor (VEGF) expression.
Case Report
The patient is a 57-year-old man status postorthotopic heart transplantation for ischemic cardiomyopathy. Routine cardiac biopsy results were normal until 5 months after the transplantation when mild rejection was noted (grade I). One month later, he experienced altered mentation concerning for tacrolimus toxicity, rejection, or infection. Empiric antibiotics and antiviral agents were initiated, but results of work-up for bacterial and virus infections, including lumbar puncture and blood cultures, were negative, and serum tacrolimus levels were within normal limits (13.2 ng/mL; laboratory upper limit of normal, 16 ng/mL). The patient clinically improved and was discharged to home.
One week later, he again presented with confusion. Results of repeated blood cultures and lumbar puncture were negative. On day 5, he experienced a grand mal seizure. At that time, white blood cell count was 3.4 × 103 μL−1 (77% polymorphonuclear leukocytes, 8% bands, 10% lymphocytes), platelet count was 190 × 103 μL−1, and serum glucose level was 80 mg/dL. Emergent outside CT suggested a “subacute stroke,” and he was transferred to the University Hospital for further management.
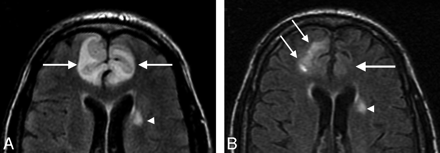
On transfer, the patient was lethargic and febrile (102.1°F) but with normal blood pressure readings (range, 114–138/41–66 mm Hg). Brain MR imaging examination demonstrated bifrontal signal intensity abnormality (Fig 1A) consistent with vasogenic edema without restricted diffusion or infarction. Concern remained high for underlying infection; antibiotics were reinstated.
MR imaging studies of reversible encephalopathy. A, Axial MR fluid-attenuated inversion recovery image obtained at toxicity demonstrates vasogenic edema in the frontal lobes bilaterally (arrows). Minor small-vessel disease changes were also present (arrowhead). Diffusion sequence demonstrated T2 shinethrough, but true restricted diffusion was not present. B, Axial MR fluid-attenuated inversion recovery image obtained 1 month after right frontal lobe biopsy demonstrates near-complete resolution of the edema in the left frontal lobe (large arrow). Postbiopsy changes are present in the right frontal lobe (small arrows) with resolution of the previously present vasogenic edema deep to the biopsy changes. Residual small-vessel disease changes are again noted (arrowhead).
After 6 days, some clinical improvement was noted, but seizure activity persisted and a second MR imaging examination continued to demonstrate bifrontal edema. A positive stool toxin for Clostridium difficile was identified, but results of comprehensive infection work-up (blood/urine/CSF) remained otherwise negative. Concern at this point included undefined brain infection or possible posttransplant lymphoproliferative disease, and it was elected to perform a biopsy of the frontal lobe lesion on the right.
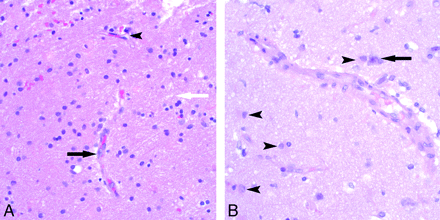
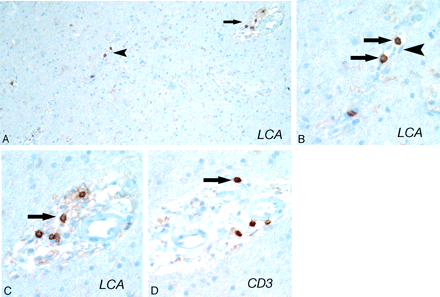
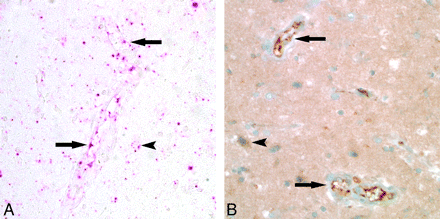
Histologic examination demonstrated evidence of endothelial activation, diffuse vasogenic edema, reactive astrocytes, and reactive microglia (Fig 2A and B). Results of leukocyte common antigen stain demonstrated T-cell (CD3 antigen positive) specific trafficking with intravascular, transmural, and perivascular T-lymphocytes (Fig 3). T-cell trafficking was nonselective with both helper (CD4) and cytotoxic (CD8) subtypes represented. Results of specific antibody staining VEGF and in situ hybridization for VEGF messenger RNA were also positive (Fig 4).
Early microscopic pathologic features of reversible encephalopathy, hematoxylin-eosin stain. A, Hematoxylin- eosin–stained sections showed many small blood vessels with reactive endothelial cells (black arrow) in the white matter demonstrating cellular and nuclear enlargement as well as mild interstitial edema (white arrow). Some vessels appeared nonreactive and are visible as a comparison (arrowhead). No vasculitis, demyelination, or axonal swelling was seen. Some scattered erythrocytes are present from the biopsy procedure, but no hemosiderin-laden macrophages were seen. B, These reactive blood vessels were also present in the gray matter, seen adjacent to a neuron (arrow), along with reactive astrocytes (arrowheads).
Early microscopic pathologic feature of reversible encephalopathy on immunohistochemical examination. A, CD45 (leukocyte common antigen) shows scattered lymphocytes in the intravascular (arrowhead) and perivascular (arrow) areas. The vessels identified by the arrows are shown in higher magnification in Figs B and C. B, Higher magnification of arrowhead in Fig 3A. Lymphocytes (T-cells) are regularly noted within the intravascular space (arrows) surrounded by endothelium (arrowhead). Other leukocytes (polymorphonuclear leukocytes, monocytes, macrophages) were not identified, suggesting selective T-lymphocyte adherence. C, Higher magnification of arrowhead in Fig 3A. Lymphocytes (T-cells) have traversed the endothelium (end point of the lymphocyte trafficking process) and are in the perivascular space (arrow). D, These lymphocytes were CD3-positive T-cells (arrow). No CD20-positive B-cells were present, and CD68 immunostaining identified only a modest number of activated microglia with no macrophages (not shown). Results of immunohistochemical and in situ hybridization studies were negative for toxoplasmosis, herpes simplex virus 1 and 2, Epstein-Barr virus, and JC virus (not shown). Results of tissue cultures were also negative.
VEGF expression in reversible encephalopathy. A, In situ hybridization with a probe specific to VEGF messenger RNA showed robust transcription (red pigment) in the endothelium (arrows), astrocytes (arrowhead), and neurons. Results of biopsy of control brain tissue were negative (not shown). B, Immunohistochemical study for VEGF protein expression showed strong staining (deep brown pigment) in the brain tissue, including the endothelium (arrows), astrocytes (arrowhead), and neurons.
Trafficking leukocytes were not B-cells (CD20), and no polymorphonuclear cells, monocytes, or macrophages were identified on hematoxylin-eosin sections. Focal lymphocyte accumulation was not present.
Tacrolimus was empirically switched to cyclosporine, the patient's mental status continued to improve, and he was discharged to home. Follow-up MR imaging examination 1 month after discharge (Fig 1B) demonstrated near-complete reversal of the bifrontal vasogenic edema.
Discussion
Because most cases of reversible encephalopathy are diagnosed solely by clinical and radiologic findings, reports describing histologic findings in the acute phase of toxicity are not common. Of those, some are in the postmortem setting where the pathologic process was severe; in such cases, diffuse white matter edema with thrombotic microangiopathy, ischemia, and perivascular lymphocytic infiltration were described.1,2 Reports of biopsy being performed on material in the premortem setting have generally shown vasogenic white matter edema, perivascular lymphocytes, reactive astrocytosis,3–6 and perivascular macrophages.7,8 Endothelial immunoreactivity to tumor necrosis factor-alpha has been demonstrated.4 Some white matter pallor has been attributed to demyelination,9 but this feature more likely represents focally pronounced edema.4 The case of our patient suggests that endothelial activation (with subsequent edema) and perivascular trafficking of T-cells play important roles in the pathogenesis of reversible encephalopathy.
The classic form of reversible encephalopathy is PRES, typically demonstrating vasogenic edema in the parieto-occipital region, frontal lobes, temporo-occipital junction, and cerebellum,10 but other reversible states or presentations are recognized, including isolated brain stem or basal ganglion edema and reversible lesions in the splenium of the corpus callosum.11,12 PRES is recognized after transplantation but is also seen in other complex systemic processes including preeclampsia/eclampsia, infection/sepsis/shock, autoimmune disease, and postcancer chemotherapy. Although the mechanism is as yet unproven, the associated conditions have immune-related antigenic challenge as a common characteristic.13
The current case has elements of classic posttransplant neurotoxicity (altered mentation, reversible brain edema, recent mild rejection) lacking only the typical PRES edema distribution. Indeed, this unusual pattern was the main reason biopsy was performed. Histologic demonstration of T-cell, but not B-cell, trafficking (intravascular, transmural, perivascular T-cells) suggests selective T-cell activation, whereas VEGF (previously vascular permeability factor) expression, likewise, suggests tissue hypoxemia and is likely responsible for the vasogenic edema.
No evidence of local brain infection to account for the T-cell response was identified (histologic examination, immunohistochemical examination, tissue culture). Results of systemic testing for bacterial and virus infection were negative.
A mechanism for the developing vasogenic edema has been proposed on the basis of T-cell activation: endothelial cell activation or injury, T-cell trafficking, plus-or-minus superimposed vasoconstriction with resultant hypoperfusion or hypoxemia, VEGF expression, and increased endothelial permeability.13 Endothelial activation (histologically endothelial cell and nucleus enlargement) and selective T-cell trafficking could certainly lead to restricted brain blood flow with reduced perfusion and hypoxemia. In this case, recent low-grade rejection (reaction to transplant endothelium) or the presence of colon flora imbalance with C difficile might have been the foreign protein antigen source that led to this pathologic sequence of events. The findings in this case suggest that reversible encephalopathy after transplantation may be because of an aberrant T-cell mediated immune response.
References
- Received July 23, 2008.
- Accepted after revision August 5, 2008.
- Copyright © American Society of Neuroradiology