Abstract
BACKGROUND AND PURPOSE: The molecular characteristics of the pathophysiology of saccular aneurysms remain poorly understood. The purpose of the current study was to investigate the expression of various groups of genes at different stages of aneurysm age in elastase-induced saccular aneurysms in rabbits through the use of deoxyribonucleic acid (DNA) microarrays.
MATERIALS AND METHODS: A microarray consisting of genes related to cell adhesion, apoptosis, cell signaling, growth, inflammation, vascular remodeling, and oxidative stress was constructed by using rabbit nucleotide sequences. Elastase-induced saccular aneurysms were created at the origin of the right common carotid artery (CCA) in 12 rabbits. Two weeks (n=6) and 12 weeks (n=6) after aneurysm creation, ribonucleic acid (RNA) was isolated from the aneurysm and the control unoperated left CCA and was used for microarray experiments. Real-time polymerase chain reaction (RT-PCR) was performed for validation of microarray results.
RESULTS: Of 209 genes, 157 (75%) at 2 weeks and 88 (42%) at 12 weeks demonstrated statistically significant differential expression between aneurysm tissue and the control left CCA tissue (P < .05). Multiple genes implicated in vessel wall remodeling were found to be elevated at 2 weeks and at 12 weeks. Expression of cell adhesion molecules and antioxidant enzymes was down-regulated at 2 weeks but was not significantly different from that of controls at 12 weeks. Most transcription factors, inflammatory genes, and structural genes showed underexpression at both time points. The expression profiles of selected genes were confirmed by RT-PCR.
CONCLUSION: Multiple genes in diverse pathways have been differentially expressed in the rabbit aneurysm model.
Intracranial aneurysms are complex lesions associated with multiple etiologic factors.1 Many risk factors have been identified, but the molecular events responsible for the formation, growth, and rupture of cerebral saccular aneurysms remain unknown. Differential expression of many genes and proteins, including matrix metalloproteinases and their inhibitors, cathepsins, caspases, and proinflammatory molecules, have been implicated in the pathobiology of human intracranial aneurysms.2-5 Small previous studies have focused on involvement of individual genes or proteins in the progression and rupture of aneurysms. Recently, microarray analysis studying global gene expression of a human intracranial aneurysm was reported.6
The development of gene-chip complementary deoxyribonucleic acid (DNA) microarrays has been made it possible to examine simultaneous expression of thousands of gene products in the same experiment. Microarray techniques have been applied to diverse disease processes, yielding valuable insights into the pathologic profiles of altered gene expression in these conditions.7,8
The elastase-induced preclinical model of a human saccular aneurysm has been widely applied to study the growth of aneurysms and to analyze the healing of aneurysm after endovascular procedures.9-13 Our group previously reported the use of a rabbit-specific gene chip to identify the differential expression of genes in the experimental aneurysms at a single time point.14 We believe that global analysis of multiple genes will provide information useful in identifying important functional pathways involved in the pathobiology of aneurysms. In the present study, we extended our previous work and applied the microarray technology to study the global expression of genes at 2 time points following experimental aneurysm creation, to study temporal changes in multiple pathways potentially important in aneurysm pathology.
Materials and Methods
Construction of a Gene Chip
A rabbit gene chip, with the ability to detect the expression of 209 genes, was constructed as described in our prior article.14 Briefly, we selected approximately 400 genes that are potentially related to the pathophysiology of human intracranial aneurysms and abdominal aortic aneurysms. Of the 400 genes of interest, we identified 209 rabbit genes that had been sequenced and posted on the GenBank data base. The oligonucleotides of genes of interest were constructed commercially and spotted on the microarray slides (Operon, Huntsville, Ala).
Aneurysm Creation
The Institutional Animal Care and Use Committee approved all procedures before initiation of the study. Elastase-induced saccular aneurysms were created in 12 New Zealand white rabbits (body weight, 3–4 kg) by using the rabbit elastase model. Detailed procedures for aneurysm creation have been described in depth elsewhere.15 Briefly, anesthesia was induced with an intramuscular injection of ketamine, xylazine, and acepromazine (75, 5, and 1 mg/kg, respectively). Using a sterile technique, we exposed the right common carotid artery (RCCA) and ligated it distally. A 1- to 2-mm beveled arteriotomy was made, and a 5F vascular sheath was advanced retrograde in the RCCA to a point approximately 3 cm cephalad to the origin of RCCA. A 3F Fogarty balloon was advanced through the sheath to the level of the origin of the RCCA with fluoroscopic guidance and was inflated with iodinated contrast material. Porcine elastase (Worthington Biochemical, Lakewood, NJ) was incubated within the lumen of the CCA above the inflated balloon for 20 minutes, after which the catheter, balloon, and sheath were removed. The RCCA was ligated below the sheath entry site, and the incision was closed.
Tissue Harvest
Aneurysm samples were harvested either at 2 weeks (n=6) or 12 weeks (n=6) following aneurysm creation. Under general anesthesia, animals were euthanized by a lethal injection of sodium pentobarbital. The whole aneurysm and the contralateral CCA were dissected free from the surrounding tissues and were then immediately frozen in liquid nitrogen.14 The frozen tissues were stored at −70°C until they were ready for ribonucleic acid (RNA) extraction.
RNA Extraction
RNA was isolated from frozen tissues by using RNeasy Fibrous Tissue Mini Kit (Qiagen, Valencia, Calif). The quantity of the RNA was measured by using spectrophotometry, and the integrity of the RNA was confirmed by electrophoretic separation by using the 2100 Bioanalyzer (Agilent Technologies, Palo Alto, Calif).
Fluorescent Complementary RNA Synthesis
The Low RNA Input Fluorescent Linear Amplification Kit (Agilent Technologies) was used to synthesize complementary DNA (cDNA) and then fluorescent complementary RNA (cRNA).16 Total RNA (1 μg) was amplified and synthesized into 2 μg of cRNA. The amplification technique was tested to confirm proportional amplification of each RNA strand. cRNA was then labeled with red (Cy3) and green (Cy5) fluorescent dyes for the purpose of the microarray experiment.
Microarray Hybridization
Two micrograms of the aneurysm cRNA (labeled with the fluorescent red tag) and 2 μg of the CCA cRNA (labeled with the fluorescent green tag) were then mixed and hybridized to the microarray. The colors were switched and the hybridization was repeated in duplicate to control for the effect of the dye tag on hybridization.
Scanning
The microarray slides were then scanned, and the computer reported the intensity of the red and green fluorescent dyes for each spot on the microarray (GenePix 4000B; Axon, Union City, Calif). For example, a red spot would mean that the aneurysm messenger RNA (mRNA) dominates, a green spot would mean that the carotid artery mRNA dominates, and a mixed color spot would mean that the mRNA is equally represented.
Quantitative Real-Time Polymerase Chain Reaction Analysis
Quantitative real-time polymerase chain reaction (RT-PCR) was used to confirm the expression of 10 different genes identified by microarray experiments. Two hundred nanograms of RNA was reverse-transcribed to cDNA by using Superscript III (Invitrogen, Carlsbad, Calif). Primers were designed by using Primer3 software,17 and quantitative PCR analysis was performed with primers specific for matrix metalloproteinase (MMP)-2, MMP-9, tissue inhibitor of MMPs (TIMP) −4, vascular cell adhesion molecule 1 (VCAM1), elongation factor-1 α, interleukin-10, collagen III, interferon gamma, troponin C, and β-actin on a Thermocycler (7900 HT; Applied Biosystem, Foster City, Calif).
Statistical Analysis
Analyses were performed by using the base-2 logarithm transform of the median signal intensity, and all analyses were conducted by using SAS Version 9 statistical software (SAS, Cary, NC). Normalization of the data was done by using 2-channel fastlo, a semi-parametric approach that corrects for intensity-dependent effects, developed by Eckel et al.12 The parametric component consisted of an additive main effect for the gene. The nonparametric component consisted of a set of nonparametric loess smoothers, 1 for each array, dye, and block combination. The normalized signal intensity for each observation was estimated by subtracting the predicted values obtained from the nonparametric component from the original base-2 logarithm transform of the median signal intensity. To test for differential expression between the aneurysm and the control arteries, we fit a mixed-effects linear model for each gene. The normalized expression values were the dependent variable in the mixed-effects linear models, array and dye were fit as fixed-effect covariants, and rabbit was included as a random effect. The t test statistics and corresponding P values were calculated from a linear contrast and were used as a measure of the mean change in expression between aneurysm groups relative to the variability. Genes with a P value < .05 were used to identify pathways for further investigation.
Results
Of the 209 genes used, 157 (75%) at 2 weeks and 88 (42%) at 12 weeks after aneurysm creation demonstrated statistically significant differential expression between the aneurysm and the control tissue (P < .05) (on-line Table).
Compared with controls, the expression of cell adhesion molecules such as integrin β, E-selectin, P-selectin, and cadherin was down-regulated at 2 weeks; no significant change was observed at 12 weeks in these genes. Among the adhesion molecules that were analyzed in this study, only the expression of VCAM1 was high at both 2 and 12 weeks (absolute value of 11.33 at 2 weeks and 4.13 at 12 weeks). Apart from increased expression of osteonectin and insulinlike growth factor-1, all other cytokines, chemokines, and growth factors exhibited decreased expression at 2 weeks compared with controls, whereas the 12-week aneurysms displayed elevated levels of interleukin-10 (P=.049), transforming growth factor-β–induced protein (P=.0049), and elongation factor-α (P=.026).
The genes involved in the blood coagulation cascade were found to be down-regulated at 2 weeks and 12 weeks. However, the absolute values of their expression ranged from 6–8 at 2 weeks compared with 2–3 at 12 weeks. Among inflammatory-response genes studied, most of the genes were down-regulated, with the exception of the calcium-binding glycoprotein osteonectin, whose expression was high at both time points (P=8.16 × 10−14 at 2 weeks and P=.00005 at 12 weeks).
Bone morphogenic proteins −2, −4, −5, and −7 (P < .05), caveolin (P=8.0 × 10−11), calmodulin (1.98 × 10−9), MAP kinase activated protein kinase (1.755287 × 10−10), and MAP kinase kinase (P=.0007), which function in cell cycle control and cell signaling, were down-regulated at 2 weeks. At 12 weeks, the expression of bone morphogenic proteins −4, caveolin, and MAP kinase kinase was different from that of controls in the group of genes involved in cell-cycle control and signal-intensity transductions.
As expected, MMPs and their inhibitor molecules were differentially expressed in aneurysms compared with controls. Increased expression of MMP-2, MMP-9, TIMP-1, TIMP-2, and TIMP-3 was observed at both time points. Two-week aneurysms were found to have higher expression of MMP-12 and lower expression of TIMP-4 compared with controls and also compared with 12-week aneurysms. Tissue-type plasminogen activator was down-regulated (3.47 × 10−14) at an early time point, and urokinase plasminogen activator was up-regulated (1.72 × 10−6) in chronic aneurysms. Protease inhibitors such as calpastatin and tissue factor pathway inhibitor were down-regulated at both time points.
Many of the antioxidant enzymes were down-regulated at 2 weeks, whereas expression of most of these same genes was not different from that of controls at 12 weeks. Most transcription factors and structural genes showed diminished expression at both time points compared with controls. Among the classical vascular molecules, expression of apolipoprotein E and endothelin-1 were upregulated only at 2 weeks and 12 weeks, respectively, and most other genes were predominantly down-regulated at 2 weeks following aneurysm creation.
Of 209 genes studied in the gene-chip experiment, only 4 genes showed expression in opposite directions between 2 and 12 weeks. Expression of TIMP-4 and collagen II-β were up-regulated at 2 weeks and down-regulated at 12 weeks, whereas expression of osteoglycine and interleukin-10 was elevated at 12 weeks and lowered at 2 weeks compared with controls.
RT-PCR
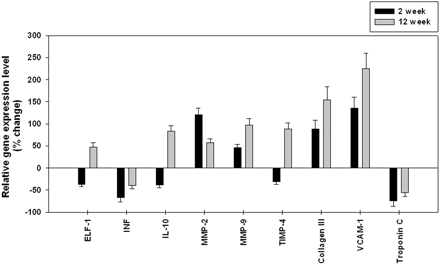
To validate the results of microarray analysis, we independently examined the pattern of mRNA expression for a series of selected genes with RT-PCR. The significance and magnitude of alterations in expression as detected by RT-PCR corresponded with the results of the microarray analysis. Figure 1 shows the expression level of genes analyzed.
Quantitative RT-PCR confirmation of differential expression in aneurysm samples for differentially expressed genes identified by microarray analysis. The relative levels of gene expression in aneurysms compared with controls. ELF-1 indicates elongation factor-1β; INF, interferon-γ; IL-10, interleukin-10.
Discussion
Many different genes and intracellular signaling pathways that control important aspects of the life cycle of a cell, including proliferation, differentiation, maintenance of ion and solute homeostasis, and apoptosis, have been implicated in arterial diseases.18-20
A rabbit gene chip was constructed with the ability to detect differences in 209 genes on the basis of the nucleotide sequences specific for the rabbit genome. Each of these candidate genes was chosen on the basis of potential involvement in processes considered key in aneurysm pathophysiology, including vessel wall remodeling, extracellular matrix degradation, vascular inflammation, transcription factors, oxidative stress, and apoptosis as described previously.14
In the present study, we have analyzed the expression of a different group of genes by using microarray technology on elastase-induced experimental aneurysmal growth at 2 and 12 weeks. This model of aneurysms mimics the hemodynamics and geometric features of human intracranial aneurysms. We acknowledge that it is not a natural pathologic aneurysm but is, instead, a surgically created artificial aneurysm model. Even within these limitations, the model may have value in understanding the expression of vital biomolecules at different stages of aneurysms.
Aneurysms are believed to be an inflammatory disorder.21 However, inflammation was rarely observed in our model in previous publications.9,15 A number of genes involved in the inflammatory response were down-regulated at both time points studied. In the present study, up-regulation in the expression of anti-inflammatory interleukin-10 was found in the 12-week aneurysm samples, which may be a reason for the absence of inflammation in this experimental aneurysm.15
The structure and function of the endothelium change as the experimental aneurysms mature. Endothelin-1 is a strong vasoconstricting protein that plays a part in vascular homeostasis. Blockage of the endothelin receptor suppresses the progression of aneurysms,22 and an elevated level of endothelin was observed in ruptured aneurysms.23 Increased expression of endothelin-1 in the 12-week aneurysm samples is in accordance with previous human studies.23
Genes of several protease family members previously implicated in the pathogenesis of cerebral aneurysms were among the genes that were differentially expressed. Aneurysms are clearly associated with accelerated degradation of the structural components of the blood vessel wall, and not surprisingly, our results confirm the importance of this process in the experimental aneurysm model. At 2 weeks, the expression of MMP-2 was higher than the expression of MMP-9, indicating the major role of MMP-2 in the aneurysm growth at the initial stage, which is in accordance with our previous study.9 Most interesting, the expression of TIMPs is also increased in aneurysm samples at both early and late time points. Tissue-type plasminogen activator was down-regulated at the early time point, and urokinase plasminogen activator was up-regulated at the later time point.
Oxidative stress has been reported to be involved in the development of elastase-induced abdominal aneurysms in rats.24 NADPH-cytochrome P450 reductase, which is related to the production of free radicals in the electron transport chain, is increased at early and later stages of aneurysm formation in our model. The expression of antioxidant enzymes, namely glutathione peroxidase and extracellular superoxide dismutase, was decreased in our experimental aneurysms at an early time point, indicating that oxidative stress may be present at an early stage of aneurysm development. The distinct expression profiles of pro-oxidant and antioxidant genes would result in an imbalance between reactive oxygen species production and free radical scavenge. As a result of the impaired redox state, oxidative damage may dilate the arterial wall and thereby accelerate the growth of aneurysms.
Clinical interventions based on these results might include specific or nonspecific inhibitors of MMPs, therapies aimed at diminishing oxidative stress or increasing growth factor or structural protein synthesis. The elastase model offers numerous advantages over surgically constructed vein-pouch aneurysm models, especially when studying biologic changes, because local suture lines are not present and the wall is arterial rather than venous. However, one must acknowledge numerous shortcomings of the elastase model when applied in gene-chip studies as in the current work. The “aneurysms” are in the mediastinum, rather than the subarachnoid space and thus subject to different perianeurysmal modulations compared with berry aneurysms. Our elastase aneurysms do not undergo spontaneous rupture, which may reflect differences in the perianeurysmal environment or may reflect fundamental differences between our experimental model and naturally occurring aneurysms. There is a “cap” of thrombus and ligated artery at the distal aspect of our experimental aneurysms, not typical for naturally occurring aneurysms. These and other dissimilarities between the elastase aneurysms and human intracranial saccular aneurysms may limit how far one can use the model system to study biologic processes, but in our opinion, the elastase model offers utility in study of such systems. We have studied the expression of only 209 genes because of the limited availability of rabbit genomic data. This microarray will be updated with new genes when new rabbit sequences are available. We have chosen the left CCA as a control, but we acknowledge that other control tissues might offer additional valuable data. These control groups would include operated nonligated RCCA vessels as well as operated ligated nondigested RCCA vessels. Future studies might be strengthened by the addition of alternative control tissues
Conclusion
Our findings demonstrate that elastase-induced aneurysms exhibit a distinct pattern of differential gene expression at different stages of aneurysm maturation. Although the functional significance of the individual gene products altered in aneurysm tissue will require further investigation, this study will be helpful to elucidate the molecular mechanism responsible for aneurysm growth.
Acknowledgments
We thank Christopher Kolbert and Vernadette Simon, Genomics Research Center, and Diane Grill, Department of Biostatistics, Mayo College of Medicine, Rochester, Minn, for their generous help with the study.
Footnotes
This work was supported by the funds from National Institutes of Health Grant 2R01NS042646.
Indicates article with supplemental on-line table.
References
- Received January 23, 2008.
- Accepted after revision March 19, 2008.
- Copyright © American Society of Neuroradiology