Abstract
SUMMARY: We report a case of incontinentia pigmenti with reversible cortex and subcortical white matter necrosis-like presentation by MR imaging. The reversible changes in follow-up imaging of the patient with incontinentia pigmenti suggest a course of natural repair of inflammation or cerebrovascular disease.
Incontinentia pigmenti (IP) is a rare X-linked dominant neurocutaneous syndrome, which primarily affects ectodermal tissues, such as the skin, eyes, teeth, and the central nervous system (CNS).1,2 Skin features are diagnostic and typically occur in 4 stages.3 We report a patient with IP with reversible extensive subcortical white matter involvement as seen on serial MR imaging, proton MR spectroscopy (1H-MR spectroscopy), and diffusion tensor imaging (DTI). Drawing from a literature review, we tried to address the possible etiology of the imaging findings.
Case Presentation
A full-term girl was born with a weight of 2550 g and Apgar scores of 10 at 1 and 5 minutes. There was no known family history of incontinentia pigmenti. A rash was found on her trunk at the twelfth day of life. Three days later, she developed seizures consisting of focal clonic jerking of her arms; each seizure lasted several minutes. She was referred to our neonatal unit because of this. Physical examination disclosed skin lesions and bilateral conjunctivitis. Her skin showed erythema, papulae, and bullae distributed on her lower limbs and trunk. Hyperpigmented macules along the Blaschko lines were present on both thighs. Hair and nails and results of routine laboratory examinations were normal. Skin biopsy demonstrated intraepidermal vesicles and spongiotic dermatitis with the presence of eosinophils and a superficial lymphocytic infiltrate. The findings supported the diagnosis of IP.
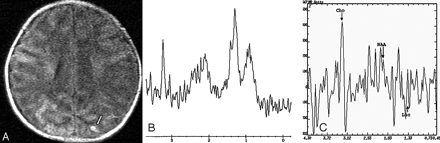
On day 19, the neonate underwent brain CT, with normal findings. MR imaging revealed a small focus of T1 signal-intensity abnormality in the left parietal lobe (Fig 1A). 1H-MR spectroscopy was performed from the right parietal lobe, which had a normal appearance on MR imaging. Single-voxel (SV) MR spectroscopy with TEs of 35 and 144 ms showed high choline (Cho), possible lactate (Lac) peak, and decreased N-acetylaspartate (NAA) (Fig 1B, -C).
MR imaging and spectroscopy at 19 days of age. T1-weighted imaging (TR = 400 ms, TE = 15 ms, section thickness = 6 mm) shows the foci signals of hemorrhage in the left parietal lobe (arrow, A). Spectroscopy with a 2 × 2 × 2 cm3 voxel located on the contralateral region of the parietal lobe, by using a point-resolved spectroscopic sequence technique (TE = 35 and 144 ms), shows a prominent Lac doublet and decreased NAA peak (B and C).
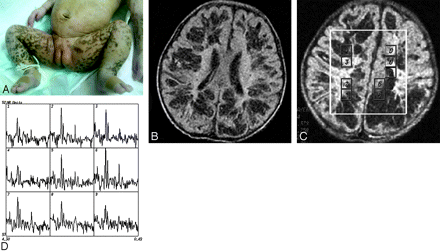
A second MR imaging was performed at day 34, when cutaneous pigmentation was most prominent (Fig 2A). This study showed extensive irregular patchy abnormalities in the periventricular and subcortical white matter in both hemispheres (Fig 2B). SV-MR spectroscopy, again in the right parietal lobe, showed findings similar to the previous one. Multivoxel MR spectroscopy with a long TE (144 ms) showed reduction of the NAA resonance and increased Cho peak (Fig 2C, -D).
MR imaging and spectroscopy at 34 days of age. Cutaneous hyperpigmented macules become more apparent along Blaschko lines in the inguinal regions (A). Fluid-attenuated inversion recovery image (B) demonstrates low signal intensity of multiple patchy softening of the subcortical white matter in the bilateral hemisphere. MR spectroscopy (multivoxel point-resolved spectroscopic sequence technique with TE = 144 ms) (C and D) in different regions shows evident reduction in NAA resonance peak amplitude at 2.02 ppm in cavities of white matter (D) (No. 4, 5 represented) and increase in the Cho peak in the adjacent parenchyma (D) (Nos. 2 and 3, 8 represented).
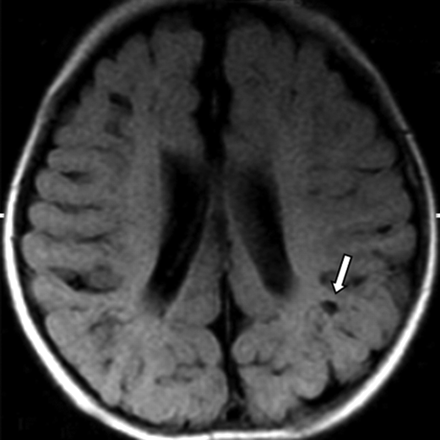
Follow-up MR imaging at 18 weeks of life revealed ventricular enlargement and multiple cystic lesions (Fig 3). To evaluate the development of white matter, we used DTI with b = 750 s/mm2 and 15 directions at day 34 and at 18 weeks. In comparison with the initial study, the latter showed fractional anisotropy (FA) values in the white matter of bilateral frontal, parietooccipital, and periventricular regions to be increased. One month later, the cutaneous lesions disappeared, and she experienced no further seizures. Mild-moderate global cognitive delay remains.
MR imaging at 4.5 months of age. Fluid-attenuated inversion recovery image reveals the disappearance of the previously observed abnormalities. However, multiple small cystic lesions exhibiting low intensity in the gray matter are present (arrow), and bilateral cerebral atrophy is also observed.
Discussion
The clinical expression of CNS involvement in IP includes mainly epilepsy and mental retardation.4,5 Ninety-two percent of patients have the characteristic skin rash by the age of 2 weeks.2 The patient in this study first presented with typical cutaneous and CNS manifestations of IP. Skin biopsy findings confirmed the diagnosis.
Our patient initially presented with a focus of high T1 signal intensity in a parietal lobe and MR spectroscopy changes suggesting ischemia. Two other articles report hemorrhagic infarcts in the periventricular white matter and associated MR angiographic findings of decreased branching of intracerebral arteries.6,7 Siemes et al8 demonstrated hemorrhagic necrosis with evidence of perivenous and capillary hemorrhage and vascular congestion in the cerebral white matter. We believe that these findings may be indicative of a microangiopathic process.
To the best of our knowledge, reversible extensive patchy abnormalities in the brain in a patient with IP as revealed by serial MR imaging, DTI, and MR spectroscopy have not been reported. In our patient, MR spectroscopy showed significant metabolic changes in these necrosis-like regions and the adjacent parenchyma. The NAA peak was decreased, and the Cho peak was increased; these changes suggested neuronal loss and possible glial proliferation. The FA values of these regions were lower when compared with normal-appearing parenchyma. When we reviewed the literature, we found that there are 2 different pathologic presentations. The first includes periventricular leukomalacia, encephalomalacia, or retinal and cerebral vascular involvement compatible with infarction.9–14 These findings support the hypothesis that vascular disease plays a major role in the pathogenesis of the CNS involvement in IP. This hypothesis is also supported by O’Doherty and Norman's neuropathologic reports.15
Different imaging features have also been recently reported.16,17 They include extensive cortical and subcortical white matter necrosis. Some lesions were cavities within the white matter accompanied by a patchy softening of the cortex. Their MR imaging appearance was similar to that of infants with acute infections of the CNS, suggesting that an inflammatory process may also contribute to the cerebral disease of IP. This hypothesis was verified by histopathology. Hauw et al18 described a diffuse inflammatory process involving the pia-arachnoid and brain, with perivascular cuffs of lymphocytes, histiocytes, and polymorphonuclear leukocytes in the white matter and cortex. Overt vascular abnormalities were absent. They argued that the brain may be involved by an inflammatory process that also results in vesicular skin eruptions. In our patient, the lesions were too extensive to be attributed to vascular occlusion. Although our patient had no signs of infection, we are unable to completely exclude encephalitis leading to encephalomalacia. The follow-up study showed an interesting appearance. MR imaging revealed the disappearance of the previously observed abnormalities. The brain looked normal except for a few residual cystic lesions in the gray matter as well as atrophy. FA values of the subcortical and centra semiovale white matter were increased compared with those in the initial study.
Yoshikawa et al19 reported a transient cavity in the white matter revealed by MR imaging in a patient with IP. They believed that the lesion was caused by a destructive process of vascular origin such as microinfarction or tissue necrosis. The reversible brain abnormalities in IP suggest a natural repair of underlying cerebrovascular disease or inflammation process.
Conclusion
Reversible extensive transient cavities in the cerebral parenchyma as seen on MR imaging, DTI, and MR spectroscopy in IP are rare. This Case Report emphasizes that the initial brain MR imaging findings in IP may simulate those caused by an infection or a microangiopathic process. IP should be included in the differential diagnosis of patients with cutaneous lesions and CNS involvement such as those seen in our patient. CNS involvement in IP might be more frequent than previously reported because of its transient nature.
Acknowledgments
We thank the patient and family for their contribution to this study. We appreciate the medical assistance of Dr. X.J. Wu of The Affiliated Children's Hospital of Zhejiang University.
References
- Received July 19, 2007.
- Accepted after revision October 16, 2007.
- Copyright © American Society of Neuroradiology