Abstract
BACKGROUND AND PURPOSE: Wernicke encephalopathy is a severe neurologic disorder that results from a dietary vitamin B1 deficiency. It is characterized by changes in consciousness, ocular abnormalities, and ataxia. This study was undertaken to analyze and compare findings on MR imaging and neurologic symptoms at clinical presentations of patients with Wernicke encephalopathy with and without a history of alcohol abuse.
MATERIALS AND METHODS: A multicenter study group retrospectively reviewed MR brain imaging findings, clinical histories, and presentations of 26 patients (14 female, 12 male) diagnosed between 1999 and 2006 with Wernicke encephalopathy. The age range was 6–81 years (mean age, 46 .6 ± 19 years).
RESULTS: Fifty percent of the patients had a history of alcohol abuse, and 50% had no history of alcohol abuse. Eighty percent showed changes in consciousness, 77% had ocular symptoms, and 54% had ataxia. Only 38% of the patients showed the classic triad of the disease at clinical presentation. At MR examination, 85% of the patients showed symmetric lesions in the medial thalami and the periventricular region of the third ventricle, 65% in the periaqueductal area, 58% in the mamillary bodies, 38% in the tectal plate, and 8% in the dorsal medulla. Contrast enhancement of the mamillary bodies was statistically positively correlated with the alcohol abuse group.
CONCLUSIONS: Our study confirms the usefulness of MR in reaching a prompt diagnosis of Wernicke encephalopathy to avoid irreversible damage to brain tissue. Contrast enhancement in the mamillary bodies is a typical finding of the disease in the alcoholic population.
Wernicke encephalopathy (WE) is an uncommon neurologic disorder classically characterized by changes in consciousness, ocular dysfunction, and ataxia.1,2 Neuroradiologic findings usually show symmetric signal intensity alterations in the mamillary bodies, medial thalami, tectal plate, and periaqueductal area.3 WE results from a deficiency of vitamin B1 (thiamine).4,5 Thiamine represents an essential coenzyme in intermediate carbohydrate metabolism but is also an osmotic gradient regulator.5 Its deficiency may cause swelling of the intracellular space and local disruption of the blood-brain barrier.6 WE represents a medical emergency, and treatment consists of intravenous administration of thiamine.1,2 The classic clinical triad of WE is not always present at clinical onset.2 Thus, diagnostic imaging modalities are useful to achieve a prompt diagnosis and treatment. In this retrospective, multicenter study, we describe the imaging findings and clinical presentations of 26 patients affected by WE because of various causes of malnutrition and malabsorption.
Patients and Methods
A multicenter study group retrospectively reviewed MR imaging studies and clinical records of 26 patients (14 female, 12 male) diagnosed between 1999 and 2006 with WE. The age range was 6–81 years (mean age, 46.6 ± 19 years). Patients were identified from a search of neurologic and neuroradiologic diagnostic data bases from various hospitals. Charts were reviewed for clinical history, symptoms at presentation, imaging modalities, and findings. Inclusion criteria consisted of a clinical diagnosis of WE and improvement at clinical presentation within 1 month from the beginning of thiamine administration. MR examinations were performed during the acute phase of the disease at a field strength of 1T (10 patients) and 1.5T (16 patients). Five of 26 (19%) MR examinations showed movement artifacts; notwithstanding, they were included in the study because they were considered to have diagnostic quality. Imaging sequences of the brain included long repetition time (TR) and short echo time spin-echo sequences, and contrast-enhanced short TR images in multiple planes. MR findings were represented by symmetric hyperintensity on T2-weighted and fluid-attenuated inversion recovery (FLAIR) images; symmetric hypointensity or no abnormalities on T1-weighted images; and symmetric areas of contrast enhancement after gadolinium injection involving the thalamus, periventricular region of the third ventricle, periaqueductal area, mamillary bodies, tectal region, and dorsal medulla. We compared the alcohol abuse group with the no alcohol abuse group on imaging findings and clinical presentation by using the 2-tailed Fisher exact test. When the test showed an association, we calculated the Phi coefficient to determine the strength of the relationship (statistical application package: SPSS version 13.0; SPSS, Chicago, Ill).
Results
Clinical Histories
Thirteen (50%) patients affected by WE had a history of chronic alcohol abuse. Thirteen (50%) patients affected by WE did not have a history of alcohol abuse. In the nonalcoholic group, the most frequent cause of thiamine deficiency was malabsorption as a result of malignant tumors of the gastrointestinal tract (6/13 patients, 46%). Among these, 4 patients underwent surgery for gastric cancer. Four (31%) patients had hyperemesis (3 with hyperemesis gravidarum, 1 with hyperemesis during chemotherapy). Three (23%) of 13 patients had severe malnutrition caused by prolonged voluntary starvation (1), anorexia nervosa (1), and socioeconomic poverty (in 1 pediatric patient).
Neurologic Findings at Clinical Presentation
The most frequent neurologic findings were changes in consciousness in 21/26 patients (81%, 10 in the alcohol abuse group [AL] versus 11 in the group with no alcohol abuse [NA]). These changes showed a wide spectrum of presentations ranging from mild disorientation to coma. Twenty patients (77%, 12 AL and 8 NA) showed ocular symptoms. Fourteen patients (54%, 10 AL and 4 NA) showed ataxia. With reference to changes in consciousness and ocular symptoms, no statistical associations were found between the alcohol abuse and the nonalcoholic groups. The alcohol abuse group showed a statistically positive correlation with ataxia (P = .047, Phi = 0.463). Only 10/26 patients (38%, 7 AL and 3 NA) showed the classic triad of the disease at clinical presentation.
Imaging Features
All 26 patients underwent MR. The MR sensibility was 0.92 ± 0.10, confidence interval = 0.95. Twenty-three (88%) patients showed high signal intensity alterations on long TR spin-echo sequences; 8 (31%) showed low signal intensity alterations on short TR spin-echo images. Twenty-two (85%) patients showed symmetric lesions of the medial thalami and the periventricular region of the third ventricle (9 AL and 13 NA). Seventeen (65%) patients showed alterations of the periaqueductal area (7 AL and 10 NA) (Fig 1). Fifteen (58%) patients showed alterations of the mamillary bodies (10 AL and 5 NA) (Fig 2). Eight (31%) patients showed alterations of the tectal plate (2 AL and 6 NA). Only 2 (8%) patients showed lesions of the dorsal medulla with selective involvement of the prepositus hypoglossal nuclei (Fig 3). Only 1 (4%) patient showed symmetric alterations of the cerebellum. Regarding the presence of signal intensity alterations in unenhanced sequences involving anatomic regions typical and atypical for the disease, no significant statistical associations were observed between the 2 groups.
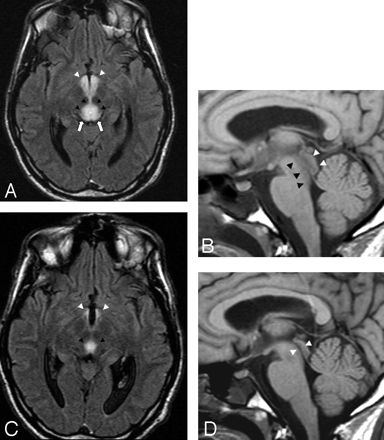
A 33-year-old man presented with sudden and progressive changes in consciousness after prolonged voluntary food starvation.
A, Axial FLAIR (11000/140/2 [TR/TE/NEX]) image shows marked hyperintensity of the tectal region (white arrows), periaqueductal area (black arrowheads), and mamillary bodies (white arrowheads). The lesions are compatible with reversible cytotoxic edema.
B, On sagittal T1-weighted (530/13/2 [TR/TE/NEX]) image, low signal intensity areas with respect to the pons of both periaqueductal areas (black arrowheads) and tectal plate (white arrowheads) are seen. Note that the local swelling determines mild stenosis of the cerebral aqueduct.
C, Ten days after thiamine replacement, a partial regression of the lesions involving the aqueductal region (black arrowheads) and mamillary bodies (white arrowheads) is easily seen on axial FLAIR image.
D, This finding is confirmed by a sagittal T1-weighted image showing reopening of the cerebral aqueduct (white arrowheads).
A 53-year-old woman with an history of chronic alcohol abuse presented with the classic neurologic triad of Wernicke encephalopathy. Coronal T2-weighted (2500/90/2 [TR/TE/NEX]) (A) and coronal FLAIR images (9000/105/2 [TR/TE/NEX]) (B) show high signal intensity circumscribed to the mamillary bodies (white arrows). After administration of contrast media, central enhancement (white arrows) of both mamillary bodies is seen on coronal T1-weighted image (532/15/2 [TR/TE/NEX]) (C).
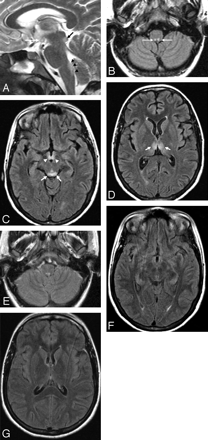
A 21-year-old woman presented with hyperemesis gravidarum, changes in consciousness, and ocular abnormalities.
A, Sagittal T2-weighted image (4447/100/4 [TR/TE/NEX]) shows high signal intensity of the lower tectal plate (black arrow), dorsal upper medulla (black arrowheads), and mamillary body (white arrow).
B, Symmetric involvement of the prepositus hypoglossal nuclei (white arrows) is demonstrated on an axial FLAIR (11000/140/2 [TR/TE/NEX]) image.
C, Alterations in the tectal plate (white arrows) and mamillary bodies (white arrowheads) are seen.
D, Axial FLAIR image at the level of the basal ganglia shows alterations of the medial thalamic nuclei (white arrows).
E-G, Twelve days after the start of thiamine replacement therapy, regression of neurologic symptoms and FLAIR abnormalities is seen.
Contrast media was administered in 17/26 (65%) patients (8 AL and 9 NA). Among these patients, 8/17 (47%, 7 AL and 1 NA) showed contrast enhancement. The anatomic structures that most frequently showed contrast enhancement were the mamillary bodies (6 patients), followed by the tectal plate (3 patients), thalamus (3 patients), and periaqueductal area (2 patients). In Patient 1 in the alcohol abuse group, the alteration seemed to be only in contrast enhancement that symmetrically involved the mamillary bodies (Fig 4). A statistically positive correlation (P = 0.002, Phi = 0.783) between contrast enhancement in the mamillary bodies and the alcohol abuse group was found. Of the group with no history of alcohol abuse, Patient 11 showed symmetric involvement of the cerebellum. Hydrocephalus due to occlusion of the cerebral aqueduct was not found in any of the patients. Data on histories, symptoms, imaging modalities, and findings in both groups are summarized in on-line Tables 1 and 2. On-line Table 3 shows the topographic distribution of lesions in both groups. On-line Figure 1 compares the groups by anatomic abnormalities. On-line Table 4 shows the neurologic symptoms typical for the disease in both groups. On-line Figure 2 compares the groups by neurologic symptoms.
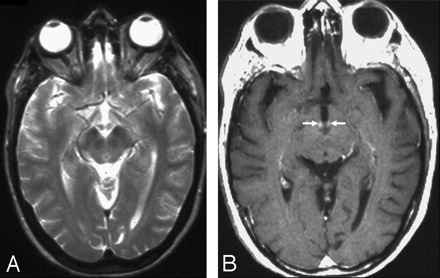
A 45-year-old woman with a history of alcohol abuse had changes in consciousness and ocular abnormalities.
A, No signal intensity alterations are seen on axial T2-weighted (2500/90/2 [TR/TE/NEX]) image at the level of the mamillary bodies and periaqueductal area. No alterations are seen on T2-weighted image at the level of the basal ganglia (not shown).
B, After administration of contrast media, contrast enhancement of the mamillary bodies is demonstrated (white arrows) on axial T1-weighted (532/15/2 [TR/TE/NEX]) image.
Discussion
WE is a life-threatening condition, and its prognosis depends on prompt diagnosis followed by intravenous administration of thiamine.2,5 If left untreated, severe amnesic deficits, Korsakoff psychosis, and even death may follow the acute phase of the disease.1,2,4,5,7 WE is frequently associated with chronic alcohol abuse, but many other pathologic conditions can cause the disease. Some of them include tumors of the gastrointestinal tract, gastroplasty for obesity, psychogenic refusal of food, hyperemesis gravidarum, anorexia nervosa, prolonged infectious-febrile disease, voluntary food starvation, chronic uremia, and parenteral therapy.2,7–13 Although the hallmark of the disease is represented by a clinical triad of altered consciousness, ocular dysfunction, and ataxia, many studies show incomplete neurologic presentation.1,2 This finding has been confirmed by our results, with only 38% of patients showing the classic triad of WE. It is noteworthy that most of them (7 AL vs 3 NA) had a history of alcohol abuse. In our study, altered consciousness, the most frequent neurologic symptom at clinical onset, was almost equally balanced between the 2 groups (AL and NA). On the other hand, ocular symptoms and ataxia were more frequently seen in the alcohol abuse group, with a positive correlation.
In our casuistry, the anatomic regions most frequently involved by symmetric lesions were the medial thalami and the periventricular area of the third ventricle. To explain these findings, we speculated that these areas represent regions where maintenance of cellular osmotic gradients is strictly related to physiologic levels of thiamine concentration and that any reduction in thiamine may determine early symmetric metabolic breakdown, as easily evidenced by MR as cytotoxic edema.5 The lesions of the thalamus and periventricular region of the third ventricle were always associated with other alterations typical of the disease (except in Patient 3 in the nonalcoholic group), which confirms a previous report.14
When sudden onset of symptoms such as somnolence and altered consciousness are present, the differential diagnosis of lesions of the symmetric medial thalami should include ischemia as a result of occlusion of the artery of Percheron and deep cerebral vein thrombosis.15–17 It is easy to understand why the ischemic diagnosis should be reasonably excluded in patients who show the association of lesions in the thalamus, periaqueductal area, tectal plate, and mamillary bodies, especially in the presence of neurologic symptoms typical of WE. Influenza A virus infection, primary acute disseminated encephalomyelitis, cytomegalovirus encephalitis, primary cerebral lymphoma, variant Creutzfeldt-Jakob disease, and West Nile virus meningoencephalitis represent other possible differential diagnoses of symmetric medial thalamic lesions.18–24
Symmetric alterations in the mamillary bodies were evident in 57% of our patients. It is noteworthy that the prevalence of lesions in the mamillary bodies was observed in the alcohol abuse group. Furthermore, we found a statistically positive correlation between contrast enhancement in the mamillary bodies and chronic alcohol consumption. We could not explain these data; we could only observe that thiamine-related deficiency from chronic alcohol abuse affected the mamillary bodies more frequently by swelling of the intracellular space and local disruption of the blood-brain barrier, compared with the patients with no alcohol abuse. In 1 patient, we found a sole alteration: contrast enhancement in the mamillary bodies, which confirmed a unique case report described previously in the English-language literature.25 A T2-weighted normal signal intensity associated with contrast enhancement in the mamillary bodies has also been described in a pediatric patient affected by WE, which also showed lesions of the periaqueductal gray matter and medial thalami.26 To explain this infrequent finding, Shogry et al25 proposed the T2-weighted “fogging effect” hypothesis previously described in cerebral infarction.27 However, this effect has been reported only in 3 patients affected by WE to date, including one of our patients with alcohol abuse and only at the level of the mamillary bodies. This finding enforces the idea that this phenomenon may be likened to the increased detection of small cortical lesions with contrast-enhanced T1-weighted images compared with the T2-weighted ones.28
We found MR findings atypical for WE in 2 nonalcoholic patients in which the dorsal medulla was involved at the level of the prepositus hypoglossal nuclei. Furthermore, symmetric hyperintense lesions of the cerebellum were seen in 1 patient. Similar atypical findings have been described by Bae et al,29 which confirmed that symmetric selective alterations of the cranial nerve nuclei and cerebellum may be seen in Wernicke encephalopathy.
Conclusion
Our study confirms that the classic neurologic triad of WE occurs in a minor part of affected patients. Thus, clinicians should suspect the diagnosis of WE, especially if changes in consciousness are present not only in alcohol abusers but also in patients with malnutrition or malabsorption, particularly if the malnutrition is the result of malignant tumors of the gastrointestinal tract and prolonged vomiting. Brain MR imaging should be included in the diagnostic flow chart of any malnourished patient with sudden onset of changes in consciousness, ocular abnormalities, and ataxia even if the symptoms are isolated. In patients with a history of alcohol abuse, contrast media should always be administered to identify lesions typical for the disease at the level of the mamillary bodies, even in the presence of normal unenhanced MR images.
Acknowledgments
We thank M. Rossi, PhD, for the statistical data revision and B. Wiley for carefully reading the manuscript.
Footnotes
Paper previously presented at: Annual Meeting of the Radiological Society of North America, December 2, 2005; Chicago, Ill.
Indicates article with supplemental on-line tables and figures.
References
- Received August 30, 2006.
- Accepted after revision November 3, 2006.
- Copyright © American Society of Neuroradiology