Abstract
BACKGROUND AND PURPOSE: Cerebral hyperperfusion syndrome is a rare but serious complication of carotid revascularization, including carotid endarterectomy (CEA) and carotid stent placement, which can occur in patients with preoperative impairments in cerebral hemodynamics. The purpose of this study was to determine whether preoperative cerebral blood volume (CBV) measured by perfusion-weighted MR imaging (PWI) could identify patients at risk for cerebral hyperperfusion after CEA.
MATERIALS AND METHODS: CBV was measured by using PWI before CEA in 70 patients with unilateral internal carotid artery (ICA) stenosis (≥70%) and without contralateral ICA steno-occlusive disease. Cerebral blood flow (CBF) was also measured by using single-photon emission CT before and immediately after CEA and on the 3rd postoperative day.
RESULTS: A significant correlation was observed between preoperative CBV and increases in CBF immediately after CEA (r = 0.785, P < .0001). Whereas hyperperfusion immediately after CEA (CBF increase of ≥100% compared with preoperative values) was observed in 7 of 15 patients (47%) with elevated preoperative CBV, no patients with normal preoperative CBV exhibited post-CEA hyperperfusion. Furthermore, elevated preoperative CBV was the only significant independent predictor of post-CEA hyperperfusion. Finally, hyperperfusion syndrome developed on the 5th postoperative day in 2 of the 7 patients who displayed hyperperfusion immediately after CEA.
CONCLUSION: Measurements of preoperative CBV by PWI might help to identify patients at risk for cerebral hyperperfusion after CEA in the absence of contralateral ICA steno-occlusive disease.
Cerebral hyperperfusion after carotid endarterectomy (CEA) is defined as a major increase in ipsilateral cerebral blood flow (CBF) following surgical repair of carotid stenosis that is well above the metabolic demands of the brain tissue.1,2 Cerebral hyperperfusion syndrome following CEA is a complication of cerebral hyperperfusion that is characterized by unilateral headache, face and eye pain, seizure, and focal symptoms that occur secondary to cerebral edema or intracerebral hemorrhage.1–4 Although the incidence of intracerebral hemorrhage is relatively low (0.4%–1.8%), the prognosis for patients with this condition is poor.2,5–9 In addition, a recent study has demonstrated that postoperative cerebral hyperperfusion, even when asymptomatic, is associated with impairment of cognitive function in patients who have undergone CEA.10
Risk factors for cerebral hyperperfusion include long-standing hypertension, high-grade stenosis, poor collateral blood flow, and contralateral carotid occlusion, which often result in impairments in cerebral hemodynamic reserve.11 Furthermore, a rapid restoration of normal perfusion pressure following CEA may result in hyperperfusion in regions of the brain in which autoregulation is impaired due to chronic ischemia. This hypothesis is similar to the “normal perfusion pressure breakthrough” theory described by Spetzler et al12 and is consistent with observations by several investigators that decreased cerebrovascular reactivity to acetazolamide is a significant predictor of post-CEA hyperperfusion.13–15
With the use of timed boluses of contrast agents, MR imaging can characterize patterns of cerebral perfusion.16–18 In fact, this method of perfusion-weighted MR imaging (PWI) can yield quantitative hemodynamic values,19–23 such as cerebral blood volume (CBV), which can be used to identify patients with hemodynamic impairment.24
The purpose of this study was to determine whether preoperative CBV measured by PWI could identify patients at risk for cerebral hyperperfusion after CEA.
Materials and Methods
Patients
Seventy patients with unilateral internal carotid artery (ICA) stenosis (≥ 70%) and useful residual function (modified Rankin Scale: 0, 1, or 2) undergoing CEA were enrolled in the present study. Sixty of the 70 patients were men. Population age was 69.0 ± 6.8 years (mean ± SD), with a range of 48–78 years of age. Concomitant disease states and symptoms were recorded, including 54 patients with hypertension. Forty-six patients showed ischemic symptoms in the ipsilateral carotid territory, and 24 patients exhibited asymptomatic ICA stenosis.
All patients underwent preoperative angiography with arterial catheterization. The overall average degree of ICA stenosis was 82.6 ± 7.9%, with a range of 70%–95%, as per the North American Symptomatic Carotid Endarterectomy Trial.25 No patient had stenosis >50% or occlusion in the contralateral ICA or middle cerebral artery (MCA).
This protocol was reviewed and approved by the institutional ethics committee, and informed consent was obtained from all patients or their next of kin.
CBV Measurements by PWI
CBV measurements, which were obtained by PWI with a Signa 3T imager (GE Healthcare, Milwaukee, Wis) and a standard head coil, were performed as described previously.24 A spin-echo–type echo-planar imaging sequence was used for PWI with a TR of 1500 ms, TE of 30 ms, FOV of 240 mm, section thickness of 7 mm, and a 128 × 128 matrix. Sixty images were obtained for each of the 7 sections. In all experiments, 12 mL of gadodiamide (Gd-DTPA-BMA; Omniscan, Nycomed Imaging, Oslo, Norway) was administered at a rate of 3 mL/s, followed by 20 mL of saline flush by using an MR-compatible power injector. The total imaging time was 90 seconds after initiation of the bolus injection.
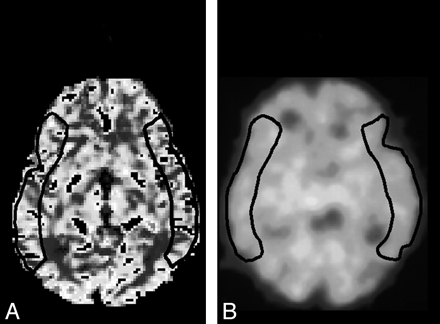
PWI data were transferred to a postprocessing workstation and analyzed by using the image analysis software system Dr. View (Asahi Kasei, Tokyo, Japan). Quantitative PWI CBV and CBF maps were generated as previously described.17–20,24 The arterial input function was obtained manually from the MCA contralateral to the CEA for deconvolution analysis. In each of the image sections, 1 large irregular region of interest was manually and bilaterally drawn in the cerebral cortex perfused by the MCA (Fig 1A), as per the atlas developed by Kretschmann and Weinrich.26 These regions of interest were placed in regions in which infarction was not present, as confirmed by T1-, T2-, or diffusion-weighted MR imaging.
Regions of interest in MCA territory selected from PW MR images (A) and SPECT (B) images.
After the CBV was determined in each region of interest, the tomographic plane with the highest CBV in the hemisphere ipsilateral to the ICA stenosis in each patient was selected and analyzed. Furthermore, the ratio of ipsilateral CBV to contralateral CBV (CBV ratio) was calculated in the selected image section in each patient to obtain the relative CBV value. The CBF was also determined in the selected image section in each patient.
Before the present study, by using the same method, 10 healthy subjects (8 men and 2 women; mean age ± SD, 60.1 ± 7.8 years of age; range, 45–75 years of age) were studied to obtain control values. The arterial input function was obtained manually from the right MCA. In each image section that passed through the basal ganglia, according to the atlas developed by Kretschmann and Weinrich,26 1 large irregular region of interest was manually and bilaterally drawn in the cerebral cortex perfused by the MCA. Thus, a total of 20 regions of interest were obtained. The control value of CBV was 4.2 ± 1.2 mL/100 g, and the control value of the CBV ratio was 1.00 ± 0.05 when the left and right sides were defined as the stenosis and contralateral sides, respectively. When the values of CBV or those of CBV ratio were more than the mean + 2 SD, (ie, 6.6 mL/100 g for CBV or 1.10 for the CBV ratio), they were rated as elevated CBV or elevated CBV ratio, respectively. The control value of CBF was 45.5 ± 12.5 mL/min per 100 g.
CBF Measurements by Single-Photon Emission CT
CBF was assessed by using iodine-123 N-isopropyl-p-iodoamphetamine (IMP) and single-photon emission CT (SPECT) before and immediately after CEA. In addition, patients with post-CEA hyperperfusion underwent a 3rd CBF measurement in the same manner, 3 days after CEA.
SPECT studies were performed by using a ring-type SPECT scanner (Headtome-SET080, Shimadzu, Kyoto, Japan), which provided 31 tomographic images simultaneously. The spatial resolution of the scanner with a low-energy all-purpose collimator was 13-mm full width at half maximum (FWHM) at the center of the FOV, and the section thickness was 25-mm FWHM at the FOV center. Image sections were taken at 5-mm center-to-center spacing, parallel to the orbitomeatal line. The images were reconstructed by using the weighted filtered back projection technique, in which the attenuation correction was made by detecting the edge of the object. Image reconstruction was performed by using an attenuation coefficient of 0.065 cm−1, a Butterworth filter (cutoff = 0.45 cycle/cm; order = 3), and a ramp filter.
The IMP SPECT study was performed as described previously, and the CBF images were calculated according to the IMP autoradiography method.27,28 In each image section obtained preoperatively and postoperatively, 1 large irregular region of interest was manually drawn in the cerebral cortex perfused by the MCA (Fig 1B), as per the atlas developed by Kretschmann and Weinrich,26 and the CBF was determined in each region of interest. These regions of interests were placed in regions in which infarction was not present, as confirmed by T1-, T2-, or diffusion-weighted MR imaging.
Post-CEA hyperperfusion was defined as a CBF increase of ≥100% (ie, a doubling) compared with preoperative values, according to Piepgras et al.2
Preoperative MR imaging including T1-, T2-, diffusion-weighted, and PW sequences and SPECT were performed more than 1 month after the last ischemic event and 7–10 days before CEA.
Intraoperative and Postoperative Management
All patients underwent surgery under general anesthesia more than 1 month after the last ischemic event. Patients were premedicated with midazolam (7.5 mg orally). Anesthesia was induced with fentanyl (2–3 μg/kg intravenously), propofol (1.5–3 mg/kg intravenously), and vecuronium (0.1 mg/kg intravenously) and maintained by repeated boluses of fentanyl (1–2 μg/kg intravenously), vecuronium, and 0.4%–1.0% inspired isoflurane. All patients were artificially ventilated with an air-oxygen mixture (inspired fraction of oxygen ∼0.30). Analysis of intermittently drawn arterial blood gas samples ensured normoventilation (4.7–5.2 kPa). Routine monitoring during anesthesia was performed by using standard electrocardiography and placement of an intra-arterial catheter for direct arterial blood pressure measurement, pulse oximetry, and capnography. Blood pressure was kept stable in a range of ±20% of the preoperative level throughout the procedure by adjusting the depth of anesthesia or, if needed, by intravenous administration of a vasodilator (nitroglycerin) or a vasoconstrictor (theoadrenalin).
An intraluminal shunt was not used in these procedures. The mean duration of ICA clamping was 32 minutes, ranging from 17 to 45 minutes. A bolus of heparin (5000 U) was given before ICA clamping, and protamine was administered at the conclusion of CEA.
All patients underwent CT imaging on the 1st postoperative day and MR imaging including T1- and T2-weighted sequences on the 3rd postoperative day to confirm the presence or absence of additional ischemic lesions.
In all patients with post-CEA hyperperfusion, intensive control of arterial blood pressure between 100 and 140 mm Hg was instituted by using intravenous administration of antihypertensive drugs immediately after SPECT. When CBF decreased and hyperperfusion resolved on the 3rd postoperative day, pharmacologic control of blood pressure was discontinued. However, when hyperperfusion persisted, systolic arterial blood pressure was maintained below 140 mm Hg. When hyperperfusion syndrome developed, the patient was placed in barbiturate coma. A diagnosis of hyperperfusion syndrome had the following requirements: 1) seizure, deterioration of consciousness level, and/or development of focal neurologic signs such as motor weakness and 2) hyperperfusion on the SPECT performed after CEA, without findings of any additional ischemic lesion on postoperative CT or MR imaging.
Statistical Analysis
Descriptive data were expressed as the mean ± SD. Correlations between preoperative CBV or CBF measured by PWI and postoperative CBF increases [(CBF calculated as a percentage of the preoperative value) − 100%] were determined by using linear and polynomial regression analyses and by computing regression equations and correlation coefficients, and the function of better fit was determined. Logistic regression analysis was used to determine the joint effect of multiple variables on hyperperfusion immediately after CEA. Covariates included age, sex, a history of hypertension, a symptomatic lesion, degree of ICA stenosis, duration of ICA cross clamp, and preoperative CBV and CBF measured by PWI. Differences were deemed statistically significance with P < .05.
Results
All patients recovered from surgery without developing new major neurologic deficits. Furthermore, patients did not exhibit additional ischemic lesions on postoperative CT and MR imaging.
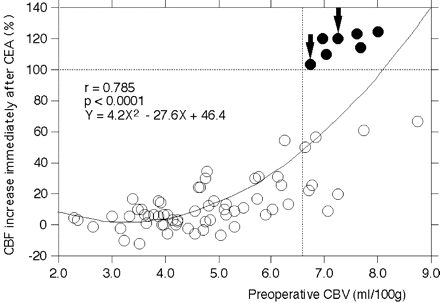
A significant square correlation was observed between preoperative CBV and the increase in CBF immediately after CEA (r = 0.785 and P < .0001) (Fig 2). Of the 70 patients studied, 7 patients (10%) met CBF criteria for post-CEA hyperperfusion on the SPECT images obtained immediately after surgery. Although post-CEA hyperperfusion was observed in 7 of 15 patients (47%) with elevated preoperative CBV, none of the patients with normal preoperative CBV (n = 55) exhibited post-CEA hyperperfusion. There was a weak linear correlation between preoperative CBF measured by PWI and the increase in CBF immediately after CEA (r = −0.324 and P = .0063). Preoperative CBF measured by PWI was reduced to the mean − 2.3 SD of the control value to the mean − 1.8 SD of the control value in the 7 patients with post-CEA hyperperfusion. Logistic regression analysis demonstrated that elevated preoperative CBV was the only significant independent predictor of developing post-CEA hyperperfusion immediately after surgery (Table). Other variables, including preoperative CBF measured by PWI, demonstrated no significant association with post-CEA hyperperfusion.
Correlation between preoperative CBV and the increase in CBF immediately after CEA. Curved line indicates square function of the best fit. Open circle indicates patients without post-CEA hyperperfusion. Closed circle indicates patients with post-CEA hyperperfusion. The arrows indicate the patients who developed hyperperfusion syndrome. The horizontal dashed line indicates CBF increase of 100% (the definition of hyperperfusion). The vertical dashed line indicates the mean + 2 SD for CBV defined in healthy subjects.
Risk factors for the development of cerebral hyperperfusion immediately after carotid endarterectomy
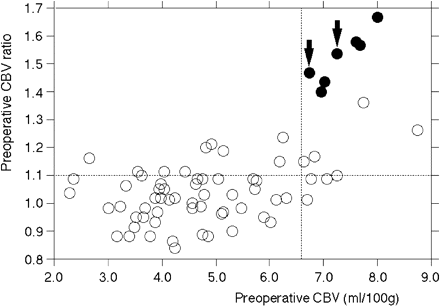
Figure 3 shows the relationship between preoperative CBV, preoperative CBV ratio, and post-CEA hyperperfusion. Although post-CEA hyperperfusion was observed in 7 of 10 patients (70%) with elevated preoperative CBV and elevated preoperative CBV ratio, none of the patients with a combination of elevated preoperative CBV and normal preoperative CBV ratio developed post-CEA hyperperfusion.
Relationship between preoperative CBV, preoperative CBV ratio, and post-CEA hyperperfusion. All 7 patients with post-CEA hyperperfusion are located in the quadrant with elevated CBV and an elevated CBV ratio. Open circle indicates patients without post-CEA hyperperfusion. Closed circle indicates patients with post-CEA hyperperfusion. Arrows indicate patients who developed hyperperfusion syndrome. Horizontal dashed line indicates the mean + 2 SD for CBV ratio defined in healthy subjects. Vertical dashed line indicates the mean + 2 SD for CBV defined in healthy subjects.
In 5 of 7 patients with post-CEA hyperperfusion immediately after surgery, hyperperfusion was not present on the SPECT performed on the 3rd postoperative day, and these 5 patients had uneventful postoperative courses. However, the remaining 2 patients with cerebral hyperperfusion immediately after CEA experienced a progressive increase in CBF on the 3rd postoperative day and developed hyperperfusion syndrome. One of these 2 patients experienced a focal seizure as evidenced by motor disturbances of the right upper extremity 5 days after surgery. The other patient experienced confusion and left motor weakness on the 5th postoperative day. Propofol coma was induced in both patients. The preoperative CBV image obtained by PWI and the pre- and postoperative CBF images obtained by SPECT for 1 of these patients are illustrated in Fig 4. Following termination of the propofol coma, both patients eventually experienced full recovery. The preoperative CBV and CBV ratio mean values of the 2 patients who developed cerebral hyperperfusion syndrome were not significantly different from those values in the other 5 patients with cerebral hyperperfusion on SPECT images obtained immediately after surgery (Fig 2).
A 66-year-old man with symptomatic right internal carotid artery stenosis (85%) exhibiting hyperperfusion syndrome after CEA. Preoperative PWI and SPECT images show elevation in CBV (A) and a decrease in CBF (B), respectively, in the right cerebral hemisphere where hyperperfusion is observed on SPECT immediately after CEA (C). This patient developed confusion and left motor weakness 5 days after surgery.
Discussion
The present study demonstrated that preoperative CBV measured by PWI might help to identify patients at risk for cerebral hyperperfusion after CEA.
Several investigators have suggested that SPECT is of utility in establishing a diagnosis of post-CEA hyperperfusion by demonstrating that all patients who developed hyperperfusion syndrome had a postoperative CBF increase of ≥100% compared with preoperative values.13–15 Furthermore, patients without postoperative CBF increase of ≥100% compared with preoperative values did not experience hyperperfusion syndrome.13–15 Thus, the present study used SPECT imaging to detect cerebral hyperperfusion and evaluated the data as a percentage of the preoperative value.
Investigators have proposed various mechanisms for the development of post-CEA hyperperfusion.4 In cases of severe ICA stenosis and deficient collateral circulation, hemispheric perfusion pressure is severely reduced distal to the ICA stenosis. This result may cause a reduction of perfusion pressure below the compensatory capacity of autoregulatory mechanisms, thus leading to maximal dilation of resistance vessels and chronic hypoperfusion or “misery perfusion.” After restoration of normal perfusion pressure following CEA, chronically impaired autoregulatory mechanisms may require several days to adjust to the new steady state, resulting in hyperperfusion in the interim. The present study demonstrated a significant square correlation between the preoperative CBV and the increases in CBF immediately after CEA. Furthermore, elevated preoperative CBV was the only significant independent predictor of post-CEA hyperperfusion. These findings support the theory that hyperperfusion results from a loss of normal vasoconstriction secondary to chronic dilation of resistance vessels and maladaptive autoregulatory mechanisms.
In the present study, although post-CEA hyperperfusion was not observed in patients with normal preoperative CBV, patients with elevated preoperative CBV did not always develop post-CEA hyperperfusion. The incidence of post-CEA hyperperfusion in patients with elevated preoperative CBV was 47%, which was lower than that in patients with reduced preoperative cerebrovascular reactivity to acetazolamide measured by SPECT (67%).15 However, additional use of the relative CBV value (eg, CBV ratio) allowed PWI to detect hyperperfusion states with a sensitivity equal to that of SPECT with acetazolamide challenge. Thus, when using PWI, one should evaluate the quantitative CBV value, as well as the ipsilateral-contralateral cerebral hemispheric asymmetry in the CBV, for a more accurate prediction of post-CEA hyperperfusion.
Several studies using SPECT have demonstrated that decreased cerebrovascular reactivity to acetazolamide is a significant predictor of post-CEA hyperperfusion.13–15 However, whether cerebrovascular reactivity to acetazolamide predicts development of hyperperfusion syndrome with a high positive predictive value remains unclear. In the present study, although hyperperfusion syndrome developed in 2 patients with a higher preoperative CBV compared with patients without hyperperfusion immediately after surgery on SPECT images, the preoperative CBV and CBV ratio mean values in these 2 patients were not significantly different from those values in the other 5 patients with cerebral hyperperfusion on SPECT images immediately after surgery. Thus, it also remains unknown if preoperative CBV can predict who will develop hyperperfusion syndrome among patients with hyperperfusion following carotid endarterectomy.
This study has several limitations. For example, the indicator dilution method with arterial input function enables quantification of hemodynamic states in the context of PWI.19,20,22,23,29,30 The arterial input function is usually obtained from the contralateral MCA in patients with unilateral ICA steno-occlusive disease,19,20,22,23,29,30 and the accurate identification of hemodynamic impairment by using CBV measured by PWI is only feasible in patients with unilateral ICA steno-occlusive disease.24 The present study also included only patients with unilateral ICA stenosis, and the arterial input function was obtained from the contralateral MCA. However, cerebral hemodynamics are more severally impaired in patients with bilateral ICA steno-occlusive disease than in those with unilateral ICA stenosis,31 and risk factors for cerebral hyperperfusion syndrome after CEA include contralateral carotid steno-occlusive disease.11 Thus, whether PWI can accurately measure CBV in patients with bilateral ICA steno-occlusive disease or whether the CBV can identify patients at risk for cerebral hyperperfusion after CEA in such patients remains unclear.
Although SPECT with an acetazolamide challenge is a reliable method for identifying patients at risk for cerebral hyperperfusion after CEA,13–15 the clinical use of SPECT is precluded by its high cost and limited availability. In addition, acetazolamide is associated with frequent various adverse effects, including metabolic acidosis, hypokalemia, numbness of the extremities, headache, tinnitus, and gastrointestinal disturbances.32 By contrast, PWI does not require ionizing radiation, and its relatively short scanning time is well suited for clinical MR imaging examinations. Although the use of the 3T MR imager in the present study is less widespread than that of the 1.5T model, CBV has been quantified by using both models.19,23,24 Furthermore, CBV measured by using 1.5 and 3T magnets reportedly correlates with cerebrovascular reactivity to acetazolamide measured by using SPECT and positron-emission tomography, respectively.24,29 Thus, CBV measured by using a 1.5T magnet might also identify patients at risk for cerebral hyperperfusion after CEA.
Most investigators recommend strict control of blood pressure in the postoperative period to prevent intracranial hemorrhage due to cerebral hyperperfusion after CEA.2–5,7–9,11,13,14 On the other hand, carotid artery disease and other vascular atherosclerotic diseases such as coronary artery disease or lower extremity atherosclerotic occlusive disease have similar risk factors and often coexist, and aggressive blood pressure control may induce ischemic events involving the other atherosclerotic steno-occlusive lesions.15 Thus, identifying patients at risk for post-CEA hyperperfusion may result in appropriate induction of postoperative blood pressure control to minimize the risk of the relative hypotension.
Conclusion
The present study demonstrated that preoperative CBV measurements by PWI might help to identify patients without contralateral ICA steno-occlusive disease who are at risk for cerebral hyperperfusion after CEA.
Footnotes
This work was supported in part by Grants-in-Aid for Science Research by the Ministry of Education, Culture, Sports, Science and Technology, Japan; and by the Mitsubishi Pharma Research Foundation.
References
- Received March 29, 2006.
- Accepted after revision July 24, 2006.
- Copyright © American Society of Neuroradiology