Abstract
BACKGROUND AND PURPOSE: Professional boxing may result in brain injury. We hypothesize that quantitative MR diffusion imaging may be useful in determining early white matter changes.
METHODS: Forty-nine professional boxers (age 30 ± 4.5 years) and 19 healthy control subjects (age 32 ± 9.5 years) were imaged on a clinical 1.5T scanner. None of the subjects had neurologic disorder or deficit. The average diffusion constant (Dav) and diffusion anisotropy (FA) were determined pixel by pixel. Regional diffusion measurements were done in the corpus callosum (CC) and internal capsule (IC). The whole brain diffusion constant (BDav) was also determined. Student t test was used to analyze the diffusion difference between boxers and the healthy control subjects. P < .05 was considered statistically significant.
RESULTS: Of the 49 professional boxers, 42 had normal conventional MRIs. The remaining 7 boxers had abnormal MR imaging findings dominated by nonspecific white matter disease. There was a significant difference in diffusion and anisotropy measurements in all the boxers compared with the healthy control subjects. In the boxer group, BDav increased and FA decreased significantly in the CC and posterior limb of IC. The measured FA and Dav inversely correlated in regions of CC and IC in boxers but not in healthy control subjects. BDav also robustly correlated with both FA and Dav in the splenium of CC in boxers.
CONCLUSION: Increased BDav and the decreased FA in the CC and IC may represent preclinical signs of subtle brain injury in professional boxers.
Professional boxing is a controversial sport, and research has raised ethical concerns1 based on biomedical findings indicating traumatic brain injury (TBI).2–12 Long-term TBI due to boxing (usually termed “dementia pugilistica,”9 “punch drunk,” and formally, chronic traumatic encephalopathy) represents a cumulative process of repetitive head blows in contact sports.2,8–10 MR imaging has been widely used to reveal brain damage including atrophy, focal or diffuse nonspecific white matter lesions, hemorrhage, infarcts, and demyelination.13,14 These microstructural changes may affect the diffusivity of water molecules within the extracellular space.15 Routine MR imaging methods may be negative or nonspecific in symptomatic patients. Diffusion tensor imaging (DTI) is very sensitive to the microscopic motion of water molecules within the extracellular space, so it can be used to characterize abnormalities of the white matter21–23 in many diseases, including early changes of TBI as seen in humans and in animal research studies.24–30 Diffusion measurements were found to be reflective of the clinical severity and prognosis of TBI,18 suggesting that diffusion parameters can be used as markers in TBI evaluation. DTI also allows quantitative analysis of the directional diffusion properties of water molecules, thus indicating the integrity of organized tissue microstructure,26,27 which is used in many situations for the measurement of the directional diffusion of white matter tracts relating to tissue orientation and integrity in white matter.28,29–31
White matter is of concern in professional boxing because of its anatomic predominance and physical function. Early detection of white matter damage may help in improving the efficacy of neuroprotective treatment of professional boxers. The purpose of this study is to find a convenient marker for the white matter changes induced by professional boxing using DTI, which may be useful in monitoring the neurologic integrity of boxers in the long run. Such a marker may also useful in evaluating patients with TBIs not caused by boxing, such as those sustained in car crashes.
Methods
Subjects
Forty-nine professional boxers (age, 30 ± 4.5 years) and 19 healthy control subjects (age, 32 ± 9.5 years) were included in this study. All the subjects were free of neurologic disease. The MR imaging was performed on a 1.5T clinical MR scanner with a quadrature head coil. Clinical MR images included: axial T1-weighted (repetition time [TR]/echo time [TE] 500 ms/minimum), axial T2-weighted (TR/TE, 4000 ms/102 ms); fluid-attenuated inversion recovery (FLAIR) (TR/TE/inversion time [TI], 10,000/162/2200 ms; matrix, 256 × 192), and diffusion-weighted imaging (TR/TE, 10,500 ms/minimum; matrix, 128 × 128; section thickness, 5 mm). There were no gaps between the sections. The conventional MR images were evaluated by the staff neuroradiologists. The study was approved by the institutional review board.
The subjects were also imaged using a single shot, echo-planar DTI sequence. Diffusion-weighted images from 26 gradient directions were acquired. An additional 6 images without diffusion weighting were also collected. The diffusion tensor was determined on a pixel-by-pixel basis. In the DTI protocol, the maximum b-value per axis was 820s/mm2. Using TR/TE, 12,000 s/100 ms, 30 contiguous sections with 5-mm thickness covering the entire brain were acquired. The FOV was 22 cm and imaging acquisition matrix was 128 × 128. The detailed methodology was reported elsewhere.26
In all subjects, a computer program was used to calculate the global BDav from the diffusion distribution histograms as reported previously.32 This program distributed the pixels into 250 bins with a bin width of 0.02 (10−5 cm2/s). This histogram was fitted to a triple Gaussian curve using commercial software (KaleidaGraph; Abelbeck/Synergy Software, Reading, Pa). The average diffusion constant (Dav) and diffusion anisotropy maps (FA) were then calculated pixel by pixel. Region of interest (ROI) measurements were done on these diffusion maps. The ROIs were drawn manually inside the structures studied. The regional Dav and FA of the genu and splenium of the corpus callosum (CC) and the anterior and posterior limbs of the internal capsule (IC) were measured (Fig 1).
Illustration of regional diffusion and anisotropy measurements with ROIs in the genu CC, splenium CC, anterior IC, and posterior IC.
Student t test was used to determine the diffusion difference between boxers and the healthy control subjects. P < .05 was considered statistically significant.
Using diffusion tensor tractography, we also visualized overall trackable white matter fibers in all subjects. We visually compared the tractography results of the boxers with those of the control subjects.
Results
Conventional MR Imaging Findings
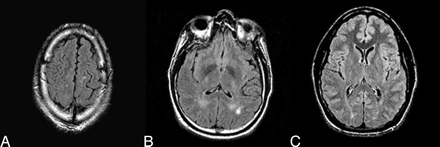
Forty-two (86%) boxers had a normal clinical MR imaging. Four (8%) had focal or diffuse nonspecific subcortical white matter disease or periventricular white matter disease, mostly in the frontal and parietal lobes. One (2%) had chronic hemorrhage, 1 (2%) had all the above, and 1 (2%) had a cavum septum pellucidum (Fig 2).
Representative axial FLAIR images of subcortical white matter disease (A), periventricular white matter disease (B), and cavum septum pellucidum (C)
Diffusion Coefficient and Anisotropy Measurements
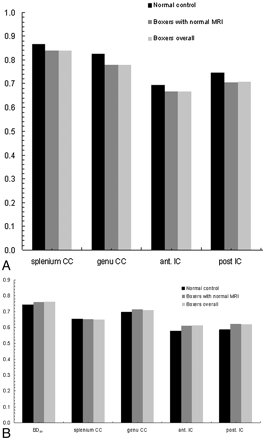
Quantitative diffusion measurements are summarized in the Table and illustrated in Fig 3. The BDav was increased by 2.6% (P < .05) in all the boxers and in 2.4% (P < .05) of boxers with normal results on MR imaging (P < .05), in agreement with a previous study done on a different boxer population.16
Comparison of FA (A) and Dav (B) measurements between boxers and healthy control subjects. Compared with healthy control subjects, boxers overall and boxers with normal MR imaging have decreased FA in splenium CC (P < .05), genu CC (P < .005), and posterior IC (P < .05) and increased BDav (P < .05) and Dav in both the anterior and posterior limbs of IC (P < .05).
Summary of diffusion measurements (in 10−5 cm2/s) in boxers and normal controls
The splenium of the CC had the highest anisotropy measured by FA, followed by genu in both boxers and healthy control subjects. The regional diffusion anisotropy measurements of all the boxers showed decreased anisotropy in the genu of the CC (6.2%, P < .005), splenium of CC (3.2%, P < .05), anterior limb of the IC (3.8%, P > .05), and posterior limb of the IC (5.4%, P < .05) compared with healthy control subjects (Fig 2). In boxers with normal MR imaging results, the regional anisotropy measurements also showed the same trend of decrease with the same significance level.
Pearson correlation analysis revealed that the regional diffusion changes of Dav significantly correlate to the corresponding FA (Fig 4A) in the genu (P < .00001), the splenium (P < .01) of the CC, and in the anterior IC (P < .001). The same analysis revealed similar robust correlations between the Dav and FA in the genu (P < .0005) and the splenium (P < .05) of CC in boxers with a normal MR imaging (Fig 4B). The correlation between FA and Dav in the anterior IC was significant (P < .05). The same analysis found no correlation between the measurements of FA and Dav in healthy control subjects. BDav was significantly correlated to the FA (negatively) and the Dav (positively) of the splenium in boxers, whereas no such correlation was found in the healthy control subjects.
Robust correlations of Dav and FA in CC of all boxers (A) and boxers with normal MR imaging (B). For the genu CC, Dav = 1.23 − 0.66 × FA, r = 0.59, P < .001; for the splenium CC, Dav = 0.95 − 0.35 × FA, r = 0.37, P < .01.
B, The group of boxers with normal MR imaging. For the genu CC (dots), Dav = 1.19 − 0.61 × FA, r = 0.55, P < .001; for the splenium CC (circles): Dav = 0.94 − 0.34 × FA, r = 0.34, P < .05.
DTI-Based Tractography
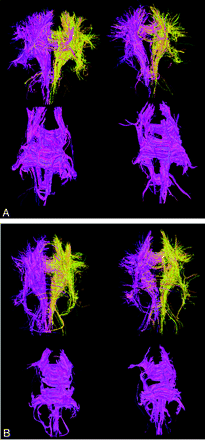
We also visualized the white matter fiber tracts over the entire brain using diffusion tensor-based tractography in all boxers. DTI-based tractography showed less trackable white matter fibers in the CC as well as the whole brain in boxers compared with healthy control subjects (Fig 5). Two boxers had repeated studies more than 6 months apart. Both repeated studies showed decreased trackable fibers in the whole brain and CC compared with their initial scans (Fig 6).
Diffusion anisotropy maps and diffusion tensor imaging-based white matter tractography of a representative boxer (A and B; 27 years old) and a control (C and D; 29 years old). Intensity is proportional to anisotropy and color shows the direction. The boxer has decreased anisotropy in CC and anterior and posterior limb of IC compared with the healthy control subjects. Fiber tracking showed overall fewer trackable white matter fibers in this boxer’s brain (B) compared with that of a control subject (D). The difference in fibers through the corpus callosum is particularly striking.
Two boxers with a follow-up study with in 464 days (A) and 658 days (B) apart, respectively. Both boxers showed decreased trackable fibers within the whole brain as well as the corpus callosum compared with the initial study.
Discussion
It is well established that active boxing exposes professional boxers to intracranial injury33 with a wide variety of pathology, including that seen in Alzheimer disease,34 though most of the imaging findings are normal or borderline.11,13,16,35,36 Professional boxers included in this study showed a variety of brain findings ranging from normal (86%) to nonspecific white matter disease mainly affecting the periventricular frontal-parietal white matter.7–10,16
In this study, we focused on the CC and IC for 2 reasons: (1) both CC and IC are oriented and tight white matter bundles, and (2) they are also the largest white matter tracts in the brain. For the diffusion analysis, in all boxers, BDav and Dav in both limbs of the IC increased significantly compared with those of the healthy control subjects. The measured increase in BDav of this study is in agreement with a previous study done on a different boxer population.16 Considering that most of the conventional MR images were normal and the abnormal conventional MR images only had nonspecific findings, this increase in measured diffusion values suggests that DTI is more sensitive than routine MR imaging in detecting the early response to brain injury induced by boxing; hence, the quantitative diffusion measurements may be used to monitor the brain.
The splenium of CC has the highest anisotropy as measured by FA followed by the genu of CC in both boxers and healthy control subjects, which agrees with a previous report.37 Well myelinated fibers and more oriented bundles may contribute to its high anisotropy.38 The robust correlation between the regional diffusion characteristics of Dav and the FA of the splenium to the BDav indicates its remarkable involvement in the brain injury, which contributes to the increased global diffusion in the boxers.
Reduced anisotropy in DTI reflects the impaired neurofilament alignment, the integrity of the myelin sheath, and axonal loss in highly oriented tissue.26,39,40 The decreased FA in the CC and posterior limb of IC in boxers overall and in boxers with normal MR imaging suggests early impairment of the axon fibers going through the CC and IC. Water diffusion is less curbed by a damaged myelin sheath. The discrepancy that diffusion did not increase significantly compared with healthy control subjects, whereas the anisotropy decreased in the CC, suggests that FA is more sensitive to the pathologic involvement of the CC in chronic traumatic encephalopathy. This discrepancy was not found in the IC in this study, though anisotropy in the IC has been reported to be sensitive in detecting other pathologic processes.41 This suggests that (1) the severity of the diffusion anisotropy changes may vary in different pathologic situations; (2) the sensitivity of anisotropy changes measured by FA is partially dependent on the intrinsic microstructural characteristics of the local anatomy; (3) the CC is more susceptible to head blows in boxing, and the diffusion changes occur earlier in this area; and (4) the diffusion anisotropy changes may provide additional markers to evaluate the boxers’ neurologic function.
In all the boxers, FA showed robust negative correlation with the corresponding Dav in the entire CC and in both limbs of IC. Unlike the boxers, no correlation was found between FA and Dav in healthy control subjects. Where fiber tracts are damaged, water diffusion is less restricted, which may result in increased Dav and decreased anisotropy.42 For the boxers with normal MR imaging results, the FA-Dav correlations are only significant in the CC. This suggests that the CC is more susceptible to the damage caused by boxing.
All boxers had decreased trackable fibers globally compared with healthy control subjects. This suggests global white matter damage. The marked decrease in the number of trackable fibers in CC suggests that CC is more susceptible to the blows to the head that characterize this sport.
Two boxers had repeat studies within 1 and 2 years, respectively. The second study of both boxers had decreased trackable fibers within the whole brain compared with their initial scans, with more marked deficit in the CC. Because we have not scanned control subjects serially during the same time period, we cannot exclude the possibility that the effects are due to experimental instability.
Conclusions
Quantitative diffusion analysis can detect diffusion anisotropy changes in the brains of professional boxers even when the clinical MR images are normal. Increased BDav may represent overall microstructural impairment of the central nervous system as a result of chronic traumatic brain injury. The anisotropy measured by FA is more sensitive than diffusion measured by Dav in reflecting earlier impairment of the white matter. The regional decreased anisotropy measurements in the boxers point to specific white matter injury. The paucity of white matter fibers on the fiber tracking points to subtle but widespread white matter damage that is not detectable on clinical MR images. Quantitative DTI shows promise as a clinical marker for early TBI in boxers. It is also expected to be a useful tool in the study of TBI in general.
References
- Received July 7, 2005.
- Accepted after revision December 22, 2005.
- Copyright © American Society of Neuroradiology