Abstract
SUMMARY: We present a variant of a split cord malformation with coexisting segmental spinal dysgenesis. CT myelography showed the left hemicord with a small remnant of subarachnoid space running through an intravertebral cleft in a spine anomaly. The left hemicord had no apparent intradual connection to the upper cord on any radiologic examination, though functional electrical stimulation studies revealed an intact efferent pathway that connected the left hemicord to the main spinal cord.
A split cord malformation (SCM), also called a diastematomyelia, is a rare spinal anomaly and refers to a sagittal division of the spinal cord into 2 symmetrical or asymmetrical hemicords. A variant of this malformation associated with a split of the spinal column, spinal bony spurs, myeloceles, myelomeningoceles, lipomas, and dermal sinuses has been previously reported in the literature.1,2 We report an unusual case of SCM with segmental vertebral anomalies.
Case Report
A 5-year-old girl was born as a low-birth-weight baby of 1190 g at 36 gestational weeks. Pregnancy and family history were unremarkable, but the short and thin left lower limb was noticed by her parents soon after discharge. She was diagnosed with SCM by MR imaging at that time, though clinical detail and MR imaging data were not available. She presented with a 5-year history of worsening neurologic dysfunction such as left leg spasticity and involuntary flexion of the left knee and was transferred to our hospital for further evaluation and treatment.
On admission, physical examination revealed a left lower limb weakness (4/5), spasticity, and atrophy. There was a small dermal sinus rostral to the anus, without communication to the intradural region. CT of the spine revealed a spina bifida at the L2 to S2 levels, and there were congenital vertebral anomalies associated with hypoplasia of T12 and L1 (Fig 1A, -B). MR imaging revealed the presence of a split cord beginning at the L2 vertebral body level in a single dural sac, and the distal thecal sac was widened (Fig 2A). A tethered cord was seen at the L5 level (Fig 2B). The right hemicord had an intradual connection to the upper cord and a relatively normal configuration. On the other hand, the proximal part of the left hemicord, with an aberrant course anterolaterally in the spinal canal, was tapered to a point of complete absence of the dural sac (Fig 2C–E) and had no apparent intradual connection to the upper cord. The distal part of the left hemicord resumed a normal appearance and presented the lateral set of nerve roots arising from the left hemicord (Fig 2F). CT myelography showed the left hemicord with the small remnant of subarachnoid space running through the intravertebral cleft at the level of spinal anomalies (T12–L1) (Fig 3). Above this region, neither the subarachnoid space nor spinal cord could be defined by CT myelography or thin-section MR images. Thus, detailed and repeated preoperative radiologic examination failed to reveal the continuity of the left hemicord to the right side and the upper cord, though there was an extradural neural tract through a canal in the deformed vertebrae (Fig 4).
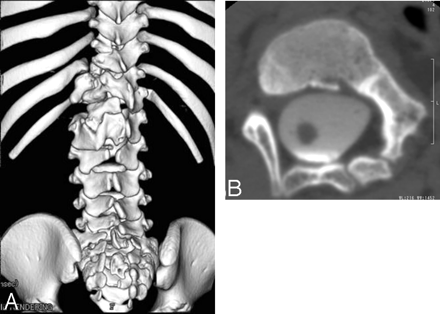
A, 3D spinal CT reconstruction of the spinal column shows spina bifida from L2 to S2 and a congenital spinal anomaly associated with hypoplasia of L1, T11, and T12.
B, CT myelography axial scan shows a congenital spinal anomaly associated with hypoplasia of L1.
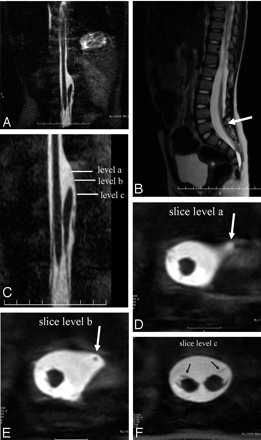
A, T2-weighted coronal MR imaging scan shows presence of split cord beginning at the T12 vertebral body level in a single dural tube, and the distal thecal sac was widened.
B, T2-weighted sagittal MR imaging scan shows the tethered cord at the level of L5 (arrow).
C, T2-weighted coronal MR imaging scan (magnified view of panel A).
D and E, T2-weighted axial MR imaging scans (section level) show the proximal part of left hemicord with an aberrant course anterolaterally to the spinal canal was tapered to a point of complete absence (arrow).
F, T2- weighted axial MR imaging scan (section level) shows the presence of lateral sets of nerve roots arising from each hemicord (arrows).
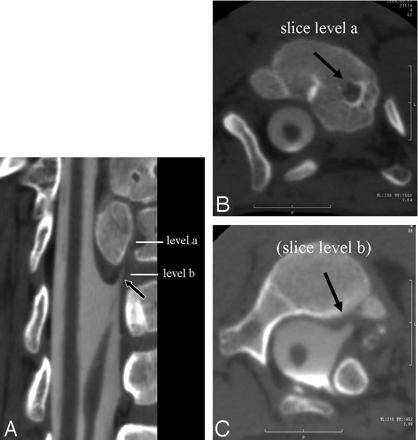
A, Curved multiplanar reconstruction image obtained with a CT myelography shows the left hemicord with the small remnant of subarachnoid space running through the intravertebral cleft at the level of spinal anomalies (arrow).
B, CT myelography axial scan (section level) shows the left small remnant of subarachnoid space running through the intravertebral cleft (arrow).
C, CT myelography axial scan (section level) shows that the proximal part of the left hemicord with an aberrant course anterolaterally and tapering (arrow).
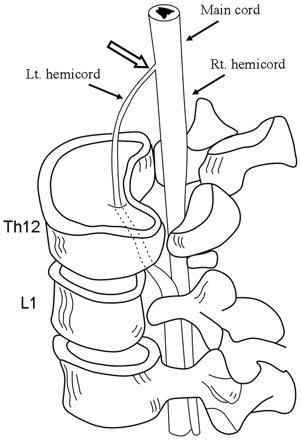
Illustration of the suspected extradural neural tract through a canal in the deformed vertebrae and the continuity of the left hemicord to the upper cord (open arrow).
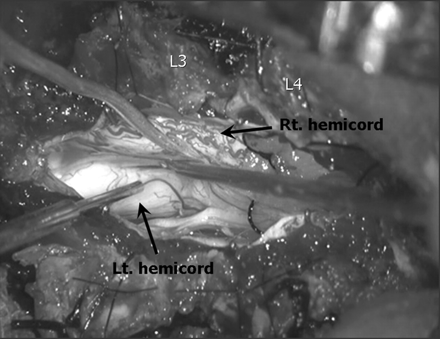
Surgery was performed to prevent the progress of the neurologic damage resulting from tethering of the cord. Surgery revealed an asymmetric duplication of the spinal cord, which was encompassed by the single dural sac, and no dural septum was recognized between either hemicord (Fig 5). Aberrant nerve roots were found along the lateral aspect of each hemicord. The proximal part of the left hemicord bent anteriorly and became smaller in diameter. Because laminectomies were performed from the level of L1 to L4, the inspection of the operating field was impossible at the spinal level of T12, and the exact intraoperative relation between the left hemicord and upper main spinal cord was not determined. Although somatosensory evoked potentials were not performed, the functional electrical stimulation studies revealed the visible movement of the left lower limb and anal muscles evoked by the electrical stimulation of the left hemicord, which suggests the efferent pathway that connects to the left hemicord from the main spinal cord. The nerve roots appeared slightly adherent to the dura at the L4–5 level, and the resection was made because the cord was tethered at the level of the lesion. The patient had an uneventful postsurgical recovery.
Photograph of the intraoperative surgical field shows an asymmetric duplication of the spinal cord, which is encompassed by the single dural sac.
Discussion
In previous reports, authors have proposed many theories to explain the embryologic basis of SCMs. Pang et al1,2 have called attention to the confusion regarding the classification of double cord malformations and proposed a unified theory to explain the embryogenetic mechanisms of all variants of SCMs. This theory is founded on the presence of anomalous neuroenteric canal and maintains that the endomesenchymal tract causes all double cord malformations. SCMs are classified as one of 2 types according to the unified theory: in type I the hemicords are always invested with individual dural sacs and the medial walls of the sacs always ensheath a rigid (bony or cartilaginous) midline spur, whereas in type II the hemicords are always within a single dural sac and the midline septum is always composed of nonrigid fibrous or fibrovascular tissues. According to this classification, our case may conform to a rare variant of type I, because 2 hemicords, each contained within its own dural sac, were separated by a rigid osseocartilaginous septum at level of T12–L1.
The spinal column in patients with SCMs has nearly always been reported to be abnormal. The lamina is often thick and fused with the ipsilateral or contralateral lamin of adjacent vertebrae, and spine bifida is almost always present. A spina bifida at the L2 to S2 levels in our case were in keeping with previous descriptions. Our case, however, differed from classic SCMs in that the vertebral body was dysplastic at the level of T12 and L1, in which a hypoplasia of the subarachnoid space and hemicord on the left was also observed. These findings radiographically similar to ours have been described as segmental spinal dysgenesis (SSD).3-5
The skeletal system develops from mesoderm.6 By about embryonic day 16, the primitive streak begins to regress and cells at the rostral lip of the primitive knot migrate between the epiblast and hypoblast, forming the notochordal process. The notochord, which develops from the notochordal process, induces surrounding mesoderm (the paraxial mesoderm, derived from the primitive streak) to condense into paired blocks of somites. Each somite becomes differentiated into a ventromedial part called the sclerotome, which will form the cartilage, bones, and ligaments of the vertebral column, and a dorsolateral part called the dermomyotome, which will form the paraspinous muscles and overlying skin. Paolo et al4 surmised that the causal insult in SSD results from chorda-mesodermal derangement during gastrulation. SSD has been reported as an autonomous entity with characteristic clinical and neuroradiologic features and a rare congenital spinal anomaly characterized by localized agenesis or dysgenesis of the lumbar or thoracolumbar spine and focal abnormalities of the underlying thecal sac, spinal cord, and nerve roots. Below the segmental agenesis, the bony spinal canal, thecal sac, and spinal cord resume a normal appearance.3
Previous studies of imaging in patients with SSD have reported that CT myelography shows marked bony narrowing of the spinal canal with the small remnant of subarachnoid space, and MR shows tapering of the spinal cord to a point of marked narrowing or complete focal absence. The radiologic findings of the left hemicord in our case were in keeping with these previous descriptions. Therefore, we concluded that our case was the rare variant of SCM with coexisting SSD.
With the assumption by Paolo et al, we thought that these unusual findings could be caused by an isolated developmental aberration and propose the following mechanism (Fig 6). Under the unified theory of Pang et al, the abnormal communication between ectoderm and endoderm causes “regional” splitting of the notochord. It would be possible that, when each separated notochord is induced surrounding the paraxial mesoderm, an embryologic derangement of the paraxial mesoderm may occur on only the left notochord. This may have stimulated the dysgenesis of left hemivertebra and hemicord. Dias et al7 showed that in complex dysraphic malformations the paired mesodermal anlagen remain separate and develop independently over variable portions of their length. Such a mechanism further supports the validity of our hypothesis. This seems to be in agreement with the very low incidence of the rare coexisting anomalies (SCM and SSD). As another mechanism, we thought that a variation in Pang’s hypothesis also might explain this unusual finding. According to the unified theory of Pang et al, in type I SCMs, the cells of the meninx primitiva pass between the split notochords and migrate around them. The sclerogenic potential of these cells leads to the formation of the midline bony spur and the hypertrophied vertebral body, and the arachnoid develops from the inner lining of those cells. Hence, the bony spur is excluded from the CSF space. During this process, it would be possible that a larger cell population accumulates along the left notochord than the right. Their sclerogenic potential might have stimulated the formation of a marked bony narrowing of left spinal canal with the small remnant of subarachnoid space.
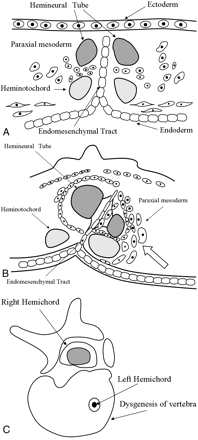
The illustrations show the mechanism of the SCMs with coexisting SSD.
A, An abnormal communication between ectoderm and endoderm causes “regional” splitting of the notochord, and each separated notochord induces surrounding the paraxial mesoderm.
B, An embryologic derangement of the paraxial mesoderm causes on only left notochord (open arrow).
C, The illustration shows the dysgenesis of vertebra and left hemicord.
- Received August 2, 2005.
- Accepted after revision September 12, 2005.
- Copyright © American Society of Neuroradiology