Abstract
BACKGROUND: Results from cerebral proton 1H-MR spectroscopy studies of neonates with perinatal hypoxic-ischemic injury have generally been presented as metabolite peak-area ratios, which are T1- and T2-weighted, rather than absolute metabolite concentrations. We hypothesized that compared with 1H-MR spectroscopy peak-area ratios, calculation of absolute metabolite concentrations and relaxation times measured within the first 4 days after birth (1) would improve prognostic accuracy and (2) enhance the understanding of underlying neurochemical changes in neonates with neonatal encephalopathy.
METHODS: Seventeen term infants with neonatal encephalopathy and 10 healthy controls were studied at 2.4T at 1 (1–3) and 2 (2–4) (median [interquartile range]) days after birth, respectively. Infants with neonatal encephalopathy were classified into 2 outcome groups (normal/mild and severe/fatal), according to neurodevelopmental assessments at 1 year. The MR spectroscopy peak-area ratios, relaxation times, absolute concentrations, and concentration ratios of lactate (Lac), creatine plus phosphocreatine (Cr), N-acetylaspartate (NAA), and choline-containing compounds (Cho) from a voxel centered on the thalami were analyzed according to outcome group.
RESULTS: Comparing the severe/fatal group with the controls (significance assumed with P < 0.05), we found that Lac/NAA, Lac/Cho, and Lac/Cr peak-area ratios increased and NAA/Cr and NAA/Cho decreased; Lac, NAA, and Cr T2s were increased; [Lac] was increased and [Cho], [Cr], and [NAA] decreased; and among the concentration ratios, only [Lac]/[NAA] was increased. Comparison of the normal/mild group with controls revealed no differences in peak-area ratios, relaxation times, or concentration ratios but decreased [NAA], [Cho], and [Cr] were observed in the infants with normal/mild outcome. Comparison of the normal/mild and severe/fatal groups showed increased Lac/NAA and Lac/Cho and decreased NAA/Cr and NAA/Cho peak-area ratios, reduced [NAA], and increased Lac T2 in the infants with the worse outcome.
CONCLUSIONS: Metabolite concentrations, in particular [NAA], enhance the prognostic accuracy of cerebral 1H-MR spectroscopy—[NAA] was the only measurable to discriminate among all (control, normal/mild, and severe/fatal outcome) groups. However, peak-area ratios are more useful prognostic indicators than concentration ratios because they depend on metabolite concentrations and T2s, both of which are pathologically modulated. Concentration ratios depend only on the concentrations of the constituent metabolites. Increased Cr T2 may provide an indirect marker of impaired cellular energetics, and similarly, NAA T2 may constitute an index of exclusively neuronal energy status. Our recommendation is to collect data that enable calculation of brain metabolite concentrations. However, if time constraints make this impossible, metabolite peak-area ratios provide the next best method of assigning early prognosis in neonatal encephalopathy.
Intrapartum hypoxia-ischemia in term infants remains an important cause of acute neonatal encephalopathy and permanent brain injury,1 affecting approximately 2 per 1000 births in the developed world.2,3 In the last 20 years, phosphorus (31P) and proton (1H) MR spectroscopy have enabled characterization of the timing and pattern of acute brain metabolite changes following perinatal hypoxia-ischemia.4-14
Clinical and experimental 31P-MR spectroscopy studies have demonstrated a biphasic pattern of impaired cerebral energy generation as a consequence of transient hypoxia-ischemia.4,7,12,14-17 The initial phase of energy generation impairment during hypoxia-ischemia (reduced phosphocreatine [PCr], adenosine triphosphate [ATP], and intracellular pH [pHi]) resolves on resuscitation but is followed by a delayed secondary phase of apparent energy failure, termed “secondary energy failure,” with characteristics similar to those found during transient hypoxia-ischemia but accompanied by a rise in brain pHi.
1H-MR spectroscopy has shown that cerebral lactate (Lac) rises during hypoxia-ischemia but is rapidly cleared on resuscitation,18 only to be followed by a secondary increase after 12–24 hours8,19 because of renewed production of Lac in brain tissue rather than entry via the circulation.20 In the basal ganglia, an increased Lac/total creatine (Cr; ie, creatine plus PCr) peak-area ratio provides an early indication of the severity of brain injury in neonates following perinatal hypoxia-ischemia, before changes are apparent on conventional longitudinal and transverse relaxation time (T1 and T2, respectively)-weighted MR imaging. A number of groups have shown that Lac/Cr, Lac/N-acetylaspartate (NAA), or Lac/choline–containing compounds (Cho) peak-area ratios provide accurate prognostic markers of the severity of brain injury and subsequent neurodevelopmental outcome.5,8-10,11-12,21-23 Indeed, such ratios may be more accurate than diffusion-weighted imaging (DWI) and the apparent diffusion coefficient (ADC) of brain water in assessing injury severity.21,22,24,25 Although Lac peak-area ratios apparently give an accurate prognosis, there is no consensus on the most appropriate ratio to use. For example, up to 10 days following birth, increased Lac/Cr,11,12,22 Lac/NAA,8-9 and Lac/Cho5,10,21,23 have all been described in neonates with subsequent poor neurodevelopmental outcome and evaluated as prognostic indices.
Few studies have measured absolute metabolite concentrations, expressed as millimole per kilogram wet weight of brain tissue because more complicated data-collection protocols and longer acquisition times are required.5,8-12,26,27 Although peak-area ratios provide a useful prognostic guide in the first 2 weeks following birth in neonates with neonatal encephalopathy, peak-area ratios can be influenced by a number of factors, including changes in metabolite concentrations and relaxation times. Experimental perinatal hypoxia-ischemia studies have demonstrated increased metabolite T2s during secondary energy failure,28 and these have been confirmed in neonatal encephalopathy.29 It has also been shown that the apparent Cr T2 depends on the relative concentrations of creatine and PCr, which may alter during secondary energy failure.30 Furthermore, a peak-area ratio could change as a result of a pathologic alteration in the concentration of either of the metabolites involved. It is, therefore, possible that interpretations based on peak-area ratios may be blind to additional pathologically important MR spectroscopy features.29,31-35
We hypothesized that compared with the interpretation provided by 1H-MR spectroscopy peak-area ratios, metabolite absolute concentrations and relaxation times measured within the first 3–4 days after birth would (1) improve prognostic accuracy and (2) enhance the understanding of underlying neurochemical changes in infants with neonatal encephalopathy.
Subjects and Methods
Subjects
Ethical permission was granted by the University College London Committee on the Ethics of Human Experimentation. Informed parental consent was obtained for every examination.
Seventeen neonates with a mean (±SD) gestational age (GA) of 39.0 (±1.6) weeks and a birth weight of 3.34 (±0.45) kg were studied at a median (interquartile range) age of 1 (1–3) days. These neonates had clinical histories consistent with neonatal encephalopathy, which included an altered conscious state, abnormal tone and reflexes, seizures, and poor feeding. Neonatal encephalopathy severity was graded by using Sarnat scores36: 5 neonates had stage I; 5, stage II; and 7, stage III. In 5 deliveries, there was cord prolapse or placental abruption. Ten neonates had a 1-minute Apgar score of less than 5, 12 infants had metabolic acidosis (pH < 7.1 and/or base deficit >12 mmol/L) in umbilical cord or arterial blood within 30 minutes of birth, and all neonates with neonatal encephalopathy required resuscitation at birth. Infants with major congenital malformations and metabolic or known infective diseases were excluded from the study.
Ten healthy control neonates of GA 39.0 (±1.8) weeks and birth weight 3.16 (±0.53) kg comparable to those of the neonates with neonatal encephalopathy were recruited from the postnatal ward and studied at 2 (2–4) days. They had no history of perinatal hypoxia-ischemia or other significant illness. Controls had Apgar scores of ≥9 at 1 minute and cord pH ≥ 7.31 (only available in 4 infants), and none required resuscitation. All control infants were physically and neurologically healthy at birth.
Neurodevelopmental Assessment
All neonates with neonatal encephalopathy who survived were examined at 1 year with the modified Amiel-Tison neurologic assessment37 and Griffiths developmental quotient (DQ).38 They were classified into 2 outcome groups: severely abnormal/fatal outcome, which comprised neonates who had died as a direct consequence of neonatal encephalopathy (n = 4) and those who had major impairment with disability on neurologic assessment and/or DQ < 75 (n = 3); and those with normal/mildly abnormal outcome (n = 10), who had either normal neuromotor outcome or mild abnormality on neurologic assessment.
Patient Handling
1H-MR spectroscopy was performed either during natural sleep or after sedation with oral or rectal chloral hydrate (50–75 mg/kg). Neonates were studied in a protective perspex pod with gentle head restraint. Throughout the examination, neonates were monitored by using MR-compatible pulse oximetry and electrocardiogram and supervised by an experienced neonatal pediatrician trained in clinical MR imaging safety.
MR Spectroscopy Methods
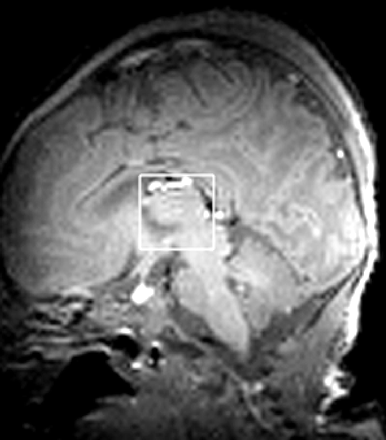
1H-MR spectra were obtained by using a 2.4T Bruker Avance system (Bruker Medizintechnik, Ettlingen, Germany) with a 40-cm clear bore, 1H radio-frequency (RF) 100.3 MHz, actively shielded gradients (Bruker S-260), and a custom-made Helmholtz head coil of 15-cm diameter and length.39 Localized 1H metabolite and water spectra were obtained by using point-resolved spectroscopy (PRESS). There was a fixed delay of 7.6 ms (much less than the echo time [TE]) between the 90° RF pulse and the first 180° pulse, thereby optimizing sensitivity for Lac. With the neonate supine, cubic 8-mL voxels were positioned. First, an axial image was obtained; then an orthogonal medial sagittal section was positioned by using the axial image; and finally, by using the sagittal image, we centered the PRESS voxel on the thalami and the midline (Fig 1). Some signal-intensity inhomogeneity was apparent on the scout images, especially anteriorly. This was due to the Helmholtz coil, which, although giving a signal intensity-to-noise ratio (SNR) of approximately 70% greater than that provided by the standard Bruker birdcage coil,39 had significantly poorer RF homogeneity. However, as PRESS RF pulse amplitudes were optimized while monitoring water signal intensity exclusively from the selected voxel which was much smaller than the Helmholtz coil, accurate 90° and 180° pulse flip angles were achieved throughout the MR spectroscopy voxel. The water signal intensity was suppressed by 3 chemical shift selection suppression (CHESS) pulses, each followed by “spoiler” magnetic-field gradient pulses.
Voxel positioning. An axial scout image was obtained and an orthogonal medial sagittal section positioned (A); the 8-mL cubic PRESS voxel was then centered on the thalami and the midline by using the sagittal image (B).
Absolute Quantitation Protocol
The absolute quantitation protocol consisted of the acquisition first of 3 partially relaxed spectra with repetition time (TR) of 2 seconds; TE 135, 270, and 540 ms; and 128, 128, and 256 summed echoes, respectively. These were followed by an essentially fully relaxed spectrum (TE, 270 ms; TR, 5 seconds; 128 summed echoes). Finally 6 non-water-suppressed fully relaxed spectra were collected (TE, 25, 270, 540, 1000, 1500, and 2000 ms; TR, 10 seconds; 8 summed echoes). Hence, the total acquisition time for the protocol (not including coil-tuning, scout imaging, voxel setup, and shimming) was about 40 minutes.
Spectrum Analysis
Echoes were baseline-corrected and exponentially filtered (1-Hz line broadening) and then zero-filled before fast-Fourier transformation. Metabolite spectra had manual phasing (0th and 1st order) and then cubic spline baseline correction. Water spectra were also manually phased (0th order only) and had linear baseline correction. Peak areas were obtained by fitting Lorentzian profiles to the spectra by using χ2 minimization with prior knowledge: starting chemical shifts, peak widths, and amplitudes; and for the Lac methyl doublet, J-coupling and equal component amplitudes.
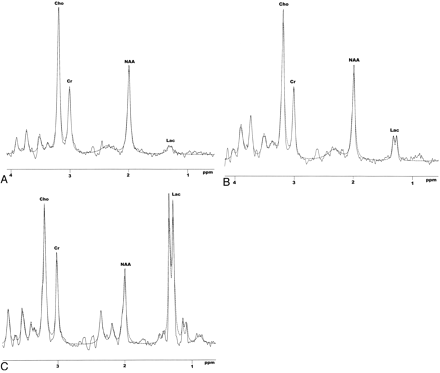
Lorentzians are solutions of the Bloch equations and should be the true line shape.40 They provided acceptable fits to Cho, Cr, and NAA. However, there was some deviation toward the base of the Lac methyl doublet, particularly when Lac was increased (Fig 2B, -C). Other researchers have used Gaussian functions, chosen empirically because they appeared to fit better. However, Cho, Cr, NAA, and Lac overlap numerous other resonances, many of which are phase-modulated multiplets with phase-inverted components at TEs of 135–540 ms. Hence, neither Lorentzians nor Gaussians provide optimal fits when other unresolved and phase-modulated components are present. (State-of-the-art analysis methods, such as AMARES,41 use exponentially decaying sinusoids [the time-domain equivalents of Lorentzians] to model the free-induction decay and, hence, will also be subject to this problem.)
Representative brain spectra from a control infant and 2 neonates with neonatal encephalopathy acquired from an 8-mL voxel centered on the thalami by using PRESS (TE, 270 ms; and TR, 2 seconds). The dashed lines are the spectrum analysis Lorentzian profiles fitted to the peaks. A, Control. B, Normal/mild outcome. C, Severe/fatal outcome.
Lac methyl doublet PRESS phase modulation presents a further problem (inversion at TE ∼135 ms; rephased at ∼270 ms and ∼540 ms). Recent work suggests 144, 288, and 576 ms give more exact phase inversion and rephasing.42 However, our results were obtained as part of a long-term study, and for consistency, TEs of 135, 270, and 540 ms were used throughout. Consequently, phase-inversion at a TE of 135 ms and rephasing at TEs of 270 and 540 ms may have been incomplete, further contributing to the slightly inexact Lorentzian fit. Again, this problem would exist regardless of the fitting function. Chemical shift artifact (spatial shift of the Lac methyl voxel relative to the methene) could have caused further incomplete phase modulation.43
Peak-Area Ratios
Lac/Cr, Lac/NAA, Lac/Cho, NAA/Cr, NAA/Cho, and Cho/Cr were measured from the TE 270 ms, TR 2 seconds spectrum.
Metabolite and Brain-Water Relaxometry
Cho, Cr, and NAA T2s were calculated, assuming monoexponential decay by using the TE 135, 270, and 540 ms spectra with a TR of 2 seconds. Incomplete phase modulation resulted in reduced Lac signal intensity with a TE of 135 ms, and hence, Lac T2 was determined by using only the TE 270 and 540 ms spectra. Because of the proximity of the lateral and third ventricles, it was possible that the PRESS voxel contained some CSF. Hence, brain-water T2 was determined by separating the signal intensity amplitudes of brain water and CSF by using a multi-TE method as described in this article.
Metabolite T1s were determined by comparing the signal-intensity amplitudes from the TE 270 ms spectra acquired with TRs of 2 and 5 seconds.
The Brain-Water Internal Concentration Reference
Brain-water was used as an internal concentration reference.29,35 To directly compare the brain metabolite signal intensity with that from brain-water, we separated the brain- and CSF-water signals. The brain-water signal intensity (T2 ∼ 150 ms) was partitioned from that of CSF (T2 ∼ 1 second) by fitting the double-exponential function SH2O.b(0) exp(-TE/T2.b) + SH2O.CSF(0) exp(-TE/T2.CSF) to the nonsuppressed multi-TE water peak areas by least-squares χ2 minimization. SH2O.b(0) and SH2O.CSF(0) were the signal-intensity amplitudes of the required brain- and CSF-water components extrapolated to TE 0 ms, and T2.b and T2.CSF, their respective relaxation times.
Quantitation of Metabolite Concentrations
Metabolite concentrations (millimole/kilogram wet tissue) were determined by comparing the T2-corrected peak areas from the fully relaxed spectrum (TE, 270 ms; TR, 5 seconds), with the fully relaxed (TR, 10 seconds) partitioned brain-water signal intensity from the same voxel and an assumed brain-water concentration. The latter was derived from previously published data for postmortem infant whole-brain: [H2Ob] = 556.0 [94.7 –0.17 GPA] mmol/kg wet weight (where GPA is the gestational plus postnatal age in weeks).44 For control infants, metabolite concentrations were calculated by using mean metabolite T2s determined from the whole group. For neonates with neonatal encephalopathy, concentrations were derived by using the metabolite T2s measured at each study. The small CSF voxel fraction in our study and the low CSF Lac concentrations reported in healthy adults and a neonatal encephalopathy subject (see Discussion) made it unnecessary to correct measured brain Lac concentrations for CSF Lac.
Data Analysis
Data between groups were compared with t tests or Mann-Whitney rank sum tests as appropriate. The level of significance was P ≤ .05.
Results
CSF
CSF voxel fractions were similar in the 3 groups of neonates: controls (mean [SD]), 5.0 (5.0)%; normal/mild, 3.9 (4.5)%; severe/fatal, 6.1 (6.0)%; and all neonates, 4.9 (5.0)%. As a consequence of the small CSF voxel fraction, the CSF T2 (1.67 [0.72] seconds) was difficult to measure and had a large SD.
Metabolite Peak-Area Ratios
In neonates with neonatal encephalopathy with severe/fatal outcome, Lac/Cho, Lac/Cr, and Lac/NAA were all increased compared with control values, and Lac/NAA and Lac/Cho were increased compared with those in infants with normal/mild outcome (Table 1). In addition, NAA/Cr and NAA/Cho in the severe/fatal outcome group were reduced compared with both control and normal/mild groups.
Metabolite peak-area ratios for Lac/Cr, Lac/NAA, Lac/Cho, NAA/Cr, NAA/Cho, and Cho/Cr
Metabolite T1 Relaxation
There were no significant differences in T1 relaxation between the outcome groups or between the outcome groups and controls (Table 2).
Spin-lattice relaxation times (T1; s) of Lac, NAA, Cho, and Cr
Metabolite T2 Relaxation
Lac, NAA, and Cr T2 were increased in the group with severe/fatal outcome compared with the control group (Table 3). Lac T2 was increased in the severe/fatal outcome group compared with that of the normal/mild outcome group. No T2 differences were found for Cho or brain water.
Spin-spin relaxation times (T2; ms) of Lac, NAA, Cho, and brain water
Metabolite Absolute Concentrations
The infants with a severe/fatal outcome showed elevated [Lac] and decreased [NAA], [Cho], and [Cr], compared with those of the control group, and lower [NAA], compared with those with a normal/mild outcome (Table 4). Neonates with neonatal encephalopathy with a normal/mild outcome also had decreased [NAA], [Cho], and [Cr], compared with those of the control group.
Absolute concentrations (mmol/kg wet weight) of Lac, NAA, Cho, and Cr
Metabolite Concentration Ratios
The only difference was in [Lac]/[NAA], which was elevated in the neonates with neonatal encephalopathy with a severe/fatal outcome, compared with that in the control group (Table 5).
Concentration ratios involving Lac, NAA, Cho, and Cr
Discussion
Comparative Prognostic Efficacies
The results of our study suggest that metabolite concentrations, in particular [NAA], are the most accurate of the MR spectroscopy measurables in neonatal encephalopathy for assigning prognosis (Tables 1–5). However, metabolite peak-area ratios, which are relaxation-weighted, are more useful prognostic indicators than relaxation times or concentration ratios. For example, whereas Lac/Cr and Lac/Cho peak-area ratios were increased in the neonatal encephalopathy group with severe/fatal outcome compared with both the control and normal/mild groups, there were no differences in [Lac]/[Cr] or [Lac]/[Cho].
Our Lac/Cr, Lac/Cho, Lac/NAA, NAA/Cr, and NAA/Cho peak-area ratios are consistent with previously published data8-12 and confirm the important role of 1H-MR spectroscopy in assigning an early prognosis of neonates with neonatal encephalopathy. Indeed, previous studies have shown that compared with qualitative or quantitative DWI, MR spectroscopy peak-area ratios were better prognostically in term infants with encephalopathy. Qualitative DWI underestimated injury extent, whereas proton MR spectroscopy on the first day after birth was predictive of outcome.24 At age 1–10 days, Lac/Cho in deep gray matter was predictive, whereas brain-water ADC was not.21
Our study showed very sensitive outcome prediction provided by the [NAA]. A preliminary study on metabolite concentrations in neonates with neonatal encephalopathy documented increases in [Lac] and reductions in [NAA], [Cho], and [Cr], similar to those reported here29; however, there were fewer infants than in the present study and limited neurodevelopmental follow-up.
Our ideal recommendation for prognosis is to measure brain metabolite concentrations. However, if time constraints or limited MR system capabilities make this procedure impossible, metabolite peak-area ratios provide the next best method of assigning early prognosis in neonatal encephalopathy.
Peak-Area and Concentration Ratio Interpretation
Although peak-area ratios are prognostically useful, our observed changes in metabolite concentrations and T2s suggest that interpretation requires caution. Pathologic changes in the concentrations and relaxation times of individual metabolites are not deducible from peak-area or concentration ratios. For example, because both the NAA and Cho concentrations and T2 relaxation are altered in neonates with neonatal encephalopathy, a fall in NAA/Cho peak-area ratio should not automatically lead the investigator to conclude that [NAA] has decreased or to estimate the magnitude of such fall. Absolute quantitation of metabolites can assess precise changes in [NAA] and [Cho], allowing a more accurate interpretation of pathologic sequelae. In particular, our quantitative results demonstrate that Cr does not constitute a stable reference for peak-area ratios. Compared with that of controls, the absolute concentration of Cr was reduced in both the normal/mild and severe/fatal outcome groups and Cr T2 was significantly increased in the latter group.
Relaxation Times
Lac, NAA, and Cr T2 were increased in the group with severe/fatal outcome compared with those in the control group. Metabolite and brain-water T2s increase in experimental models following transient hypoxia-ischemia28,45,46 and in neonates with neonatal encephalopathy.29 Our observed increase in Cr T2 in the severe/fatal outcome group would have caused pathologic modulation of Cr peak-area ratios. The increase may have resulted from 2 factors: PCr hydrolysis, leading to increased creatine and increased intracellular water, resulting in motional T2 lengthening.
The 2 major components of the Cr peak (PCr and creatine) have different T2 values (143 ms and 297 ms, respectively30). Substantial PCr hydrolysis to creatine during hypoxia-ischemia, and possibly also during secondary energy failure, would result in an apparent increase in the composite Cr T2, which, under conditions of constant Cr concentration, would increase the Cr resonance amplitude and reduce all peak-area ratios referenced to Cr. Indeed, experimental studies have shown that the T2s of Cho, Cr, NAA, and Lac all increase during both transient hypoxia-ischemia and secondary energy failure and that during secondary energy failure, these increases correlate with falling cerebral nucleotide triphosphate (mainly ATP) levels.28 A likely consequence of reduced ATP is failure of the ATP-dependent Na+/K+ pump, resulting in water influx, reduced cytosolic viscosity, and increased molecular mobility, leading to T2 lengthening.47 On the grounds of its dependence on both PCr hydrolysis and ATP levels, Cr T2 may thus be considered an indirect probe of energy metabolism.
Study Limitations
Possible limitations of our study include the time taken to acquire the data from each infant (∼40 minutes), the spatial resolution of the voxel (8 mL), and the use of a single voxel. However, if the infant is stable, then the length of examination is acceptable, considering the wealth of information obtained. The choice of voxel volume was directed by a desire to maximize SNR and spectrum resolution concomitant with acquiring data only from a limited brain region susceptible to hypoxic-ischemic injury. This choice was particularly important because the NAA concentration in neonatal brain is lower than that in adults. In addition, the NAA concentration was very low in severe neonatal encephalopathy, and because the protocol included a TE of 540 ms, we required an 8-mL voxel to collect data with sufficient SNR.
Intravoxel CSF Determination
We have not compared our CSF voxel fractions with estimates obtained from conventional MR imaging CSF/brain partitioning, first because our localized MR spectroscopy CSF fractions are essentially whole-voxel averages. Hence, to compare with conventional MR imaging, we would have to obtain many closely spaced parallel image sections covering the whole voxel. We were not able to include such an acquisition during the limited scan time available. Furthermore, a single such study would not provide statistical power for comparison. Second, the ability to separate CSF and brain-tissue pixels in conventional MR imaging is based on differences in their signal intensity amplitude, which, in turn, depend on differences in relaxation time. Hence, MR spectroscopy 2-component partitioning based on CSF and brain-water T2 and MR imaging signal intensity–amplitude partitioning are essentially founded on similar principles and should give comparable results.
Our brain-water and CSF T2 measurements justified a 2-component fit when separating CSF signal intensity from that of brain. The thalamus-centered voxel contained mainly gray matter and had an overall T2 ∼ 155 ms (Table 3). In previous studies of neonatal brain using an occipitoparietal voxel (predominantly unmyelinated white matter), we measured T2 ∼ 223 ms.35 The similarity of the thalamus-centered and occipitoparietal T2s compared with the much longer CSF T2 (∼1.7 seconds) justified fitting only a single brain component when estimating the CSF voxel fraction.
Lactate Contamination
There are 2 possible sources of MR spectroscopy signal-intensity contamination that may have resulted in overestimation of the intracellular Lac concentration. In cases of severe injury leading to significant cell death, membrane breakdown products may contribute to the Lac resonance. However, the spectrum displayed in Fig 2C (TE, 270 ms) exhibits no resonance at ∼1.6 ppm and only a small resonance at ∼0.9 ppm. If substantial liberation of fatty acids had occurred at the early stage of cerebral injury in our study, significant -CH2CH2CO- and -CH3 signals would have been seen at these respective chemical shifts.48 As a consequence of these observations, contamination of the Lac methyl resonance by signal intensity from lipid –(CH2)n- protons was probably insignificant in our studies.
CSF Lac may also be a potential contaminant. In healthy adults, CSF Lac is 0.8–2.4 mmol/L.49-51 In neonatal encephalopathy, CSF Lac concentration may be higher and was 3.7 mmol/L in a severely affected infant in the present study.52 Both CSF Lac concentration and T2 may vary unpredictably pathologically; therefore, without knowledge of these at the time of study, it is impossible to rigorously correct apparent brain Lac concentrations for CSF contamination. However, approximate estimations can be made. Assuming CSF Lac T2 ≈ brain Lac T2 (the former is probably longer), 4.9% CSF fraction (the mean for all infants in our study), and 2.4 mmol/L CSF Lac, we believe that the apparent Lac concentration would overestimate the true brain value by ∼0.12 mmol/L. Assuming the largest CSF fraction measured (19.2%) and 3.7 mmol/L CSF Lac, we believe that the apparent Lac concentration would overestimate the true brain value by ∼0.88 mmol/L. In conclusion, the effect of CSF Lac was, at the most, comparable to random error and, in general, much smaller.
Interpretation of Metabolite Concentrations and T2s
Our metabolite concentration and T2 results greatly improve pathologic interpretation compared with that provided by peak-area ratios. The putative neurochemical roles of Lac, NAA, Cho, and Cr have been discussed previously. Briefly, the elevated levels of brain Lac following transient hypoxia-ischemia are thought to be due to overproduction and/or underutilization of Lac or a change in cellular redox equilibrium in the tissue itself.18-20 Following perinatal hypoxia-ischemia, cerebral blood flow is generally increased, brain pHi can be alkaline, and there is early postasphyxial hypermetabolism53 followed by luxury perfusion in the subsequent weeks.54-56 The elevated brain [Lac] seen in injured brain following hypoxia-ischemia is probably due to a disruption of the balance between cytosolic and mitochondrial ATP-producing metabolic pathways and up-regulation of cell membrane transporters. Near-infrared spectroscopy studies suggest a decrease in oxidized cytochrome c, increased tissue oxygenation, and diminished oxygen use after hypoxia-ischemia.57-59 We speculate that the pathologic threshold for brain Lac lies between 3 and 4.4 mmol/kg wet weight (Table 4); however, a much larger study population is needed to give precise metabolite concentration sensitivities and specificities for outcome in neonatal encephalopathy.
NAA is considered a neuronal marker, because apart from its presence in oligodendrocyte type 2A (O-2A) progenitors and, to a lesser extent, in immature oligodendrocytes,60 NAA is primarily neuronal. A reduction in NAA is related to a reduction in neuronal/axonal attenuation and viability. However, because some reversibility in NAA peak-area ratios has been observed,61 it may be possible to use NAA as a marker to monitor the effects of neuroprotection or neurogenesis. Combined with the possibility of decreased neuronal attenuation from reduced NAA concentrations, the prolonged T2 implies that a proportion of the remaining intact neurons have impaired ATP generation and viability.28,29
Cho is essential in normal cell function and directly affects nerve signaling (via acetylcholine), cell signaling, lipid transport, and cell membrane metabolism.62-64 The Cho peak reflects total brain choline stores (mainly glycerophosphocholine and phosphocholine) but also has contributions from phosphoethanolamine (PEt) and ethanolamine. Cho is a putative marker of cellularity and cell turnover,65 and reduced [Cho] may be associated with delayed myelination,66 altered populations of cell types, decreased cellularity,67 or apoptosis.68-71
The Cr peak comprises signals from creatine and PCr and, to a much lesser degree, γ-aminobutyric acid, lysine, and glutathione. PCr maintains energy-dependent systems in brain cells by buffering and conserving levels of ATP and adenosine diphosphate. Reduced [Cr] in the neonatal encephalopathy groups may reflect reduced overall cellularity; the increase in Cr T2 observed in severe neonatal encephalopathy is discussed previously.
Conclusions
Our results suggest that the most important prognostic MR spectroscopy indices in neonatal encephalopathy are metabolite concentrations, in particular [NAA]. Indeed, the only measurable that demonstrated a difference among all 3 groups (control, normal/mild, severe/fatal outcome) was [NAA]—the progressive reduction in [NAA] with outcome severity suggests that neuronal attenuation and viability decline with worsening outcome. Metabolite peak-area ratios are more useful prognostic indicators than concentration ratios because they depend on metabolite concentrations and T2 relaxation, both of which are pathologically modulated—metabolite concentration ratios depend only on the concentrations of the constituent metabolites. The observed changes in metabolite concentrations and T2 values also suggest that simple interpretation of peak-area ratios needs caution because Cr, in particular, is not a stable reference. Increases in metabolite T2 values may relate to impaired cellular energy production, failure of membrane pumps, and increased intracellular water. The increase in Cr T2 may have resulted from both increased intracellular water and phosphocreatine hydrolysis, leading to increased Cr, the latter having a longer T2 than phosphocreatine. Therefore, Cr T2 may constitute an indirect index of cellular energetics. Our recommendation is to acquire data that enable calculation of brain metabolite concentrations. However, if time constraints make this procedure impossible, metabolite peak-area ratios provide the next best method of assigning prognosis in neonatal encephalopathy.
References
- Received August 30, 2005.
- Accepted after revision November 30, 2005.
- Copyright © American Society of Neuroradiology