Abstract
BACKGROUND AND PURPOSE: The vestibular nucleus cannot be visualized on MR imaging, but some patients with vestibular schwannoma show a tiny area of hyperintensity in the dorsal brain stem on T2-weighted images. The aim of this study was to determine whether this tiny area is characteristic of vestibular schwannoma.
METHODS: We retrospectively reviewed the postoperative MR images of 53 patients with cerebellopontine angle tumor. MR images were obtained with a 1.5T scanner. Spin-echo pre- and postcontrast 3-mm-thick T1-weighted axial images, 3-mm-thick fast spin-echo (FSE) T2-weighted axial images, and 0.8-mm-thick constructive interference in steady state (CISS) axial images were acquired. Surgical and histopathologic diagnosis was vestibular schwannoma (41/53 = 77%), meningioma (7/53 = 13%), epidermoid cyst (3/53 = 6%), glioma with exophytic growth (1/53 = 2%), and chordoma (1/53 = 2%).
RESULTS: A tiny area of hyperintensity was observed at the lateral angle of the fourth ventricle floor in 6 patients (3 men, 3 women; age range, 24–54 years; mean age, 43 years) with vestibular schwannoma larger than 2 cm in maximal diameter on both FSE T2-weighted and CISS images. Preoperative MR images with the same pulse sequences showed the same area of hyperintensity in all these patients.
CONCLUSION: Because the location of the area of hyperintensity is coincident with the vestibular nucleus, the hyperintensity may represent degeneration of the nucleus. This hyperintensity should not be confused with a postoperative lesion or a small infarction. If such hyperintensity is seen in a patient with a large cerebellopontine angle tumor, a diagnosis of vestibular schwannoma is suggested.
With current MR imaging techniques, it is possible to locate many of the major tracts and nuclei of the brain stem, and MR imaging is superior to other imaging techniques in delineating normal brain stem anatomy. Visualized brain stem structures on clinical MR imaging studies include the substantia nigra, inferior olivary nuclei, red nuclei, decussation of the superior cerebellar peduncles, medial lemniscus, and corticospinal tract.1,2 However, many functionally important structures, including the vestibular nuclear complex localized to the most lateral aspect of the floor of the fourth ventricle medial to the cochlear nuclei, cannot be directly visualized, and their location is inferred from external or surface structures and from identifiable internal landmarks such as those mentioned previously.2,3 On the other hand, wallerian degeneration in the corticospinal tract,4,5 secondary degeneration of the thalamus after cerebral infarction,6 and transneuronal degeneration of the limbic system in patients with temporal lobe epilepsy7 can be demonstrated as hyperintense areas on T2-weighted MR images. In clinical practice, these areas of hyperintensity should not be mistaken for other lesions such as further cerebral infarction.6
A tiny area of hyperintensity is occasionally observed on T2-weighted MR images on the affected side of the dorsal brain stem in patients with vestibular schwannoma. We reviewed pre- and postoperative MR images of patients with cerebellopontine angle tumors to determine whether the hyperintensity is characteristic of vestibular schwannoma and to confirm that the hyperintensity does not represent postoperative changes.
Materials and Methods
This retrospective study was approved by the institutional review board with a waiver of the written informed consent. The postoperative MR images of 53 consecutive patients (17 men and 35 women; age range, 13–79 years; mean age, 48.3 years) who had undergone neurosurgery for a cerebellopontine angle tumor were reviewed by 2 experienced radiologists together. Patients with neurofibromatosis were excluded. MR images were obtained with a 1.5T scanner (Magnetom Vision, Siemens Medical Systems, Erlangen, Germany), and the postoperative MR images were obtained from May 2003 through March 2004. Spin-echo pre- and postcontrast 3-mm-thick T1-weighted (TR/TE, 550/12) axial images and postcontrast T1-weighted (TR/TE, 460/12) coronal images, 3-mm-thick fast spin-echo (FSE) T2-weighted (TR/TE, 3000/90 or 3100/102) axial images, and 0.8-mm-thick constructive interference in steady state (CISS) axial images were acquired in all patients. A 3D CISS pulse sequence was used with the following parameters: TR/TE, 12.25/5.9; flip angle, 70°; field of view, 110 × 110 mm (read x-phase encoded); slab thickness, 32.0 mm; matrix, 179 × 256; 40 3D partitions; 1 slab; pixel size, 0.54 × 0.43 mm; effective section thickness, 0.8-mm; 1 signal intensity acquired; and imaging time, 5 minutes 17 seconds. The period of postoperative MR imaging examination after surgery ranged from 5 months to 11 years.
Histopathologic diagnoses of the cerebellopontine angle tumors were as follows: vestibular schwannoma (41/53 = 77%), meningioma (7/53 = 13%), epidermoid cyst (3/53 = 6%), glioma with exophytic growth (1/53 = 2%), and chordoma (1/53 = 2%). There were no schwannomas of other cranial nerves, such as facial nerve or lower cranial nerves in the study period. The preoperative MR images obtained with the same imaging protocol were available and were evaluated in 34 patients, except for 18 patients with vestibular schwannoma and 1 with meningioma.
When a tiny area of T2 hyperintensity is observed in the dorsal brain stem in patients, the depth from the floor of the fourth ventricle to the hyperintensity and the distance from the median sulcus to the hyperintensity were measured on the postoperative images, and the tumor size was measured on preoperative images. In addition, the images of 18 age-matched patients (4 men and 14 women) who underwent MR imaging with the same imaging protocol in the study period were used as a control group. The indications for MR imaging in the control group were hearing loss (12 patients), vertigo (4 patients), or ear fullness (2 patients).
Results
A tiny area of hyperintensity located at the lateral angle of the fourth ventricle from the level of the inferior cerebellar peduncle to the midpons was seen in 6 patients (Figs 1 and 2). The hyperintensity was seen on both spin-echo T2-weighted and CISS images. On postoperative images, the tiny area of T2 hyperintensity was located at the lateral angle of the fourth ventricle from the level of the inferior cerebellar peduncle to the midpons. The distance from the median sulcus to the hyperintensity ranged from 5 to 8 mm at the upper end of the hyperintensity and from 7 to 11 mm at the lower end. The depth of the hyperintensity from the fourth ventricle floor ranged from 1 to 3 mm at the upper end of the lesion and from 2 to 6 mm at the lower end of the lesion. The number of sections showing the hyperintensity was 1 or 2 on 3-mm-thick spin-echo T2-weighted images and 5 (4 cases, 67%) or 6 sections (2 cases, 33%) on 0.8-mm-thick CISS images (Table). The tiny area was not demonstrated on T1-weighted images, and no enhancement was seen after administration of gadolinium. The tiny area of T2 hyperintensity was seen on the affected side of the dorsal pons in 6 patients with vestibular schwannoma (6/41 = 14.6%; 3 men and 3 women; age range, 24–54 years; mean age, 43 years) (Fig 2). The dimensions of these vestibular schwannomas ranged from 17 × 20 × 16 mm to 39 × 50 × 36 mm. The middle cerebellar peduncle was compressed by the tumor in all 6 patients (6/6 = 100%), and deformity of the fourth ventricle was observed in 5 (5/6 = 83%). Most of the smallest tumor was solid with a few small cystic portions, and the largest tumor was composed mainly of cystic portions with a small solid portion.
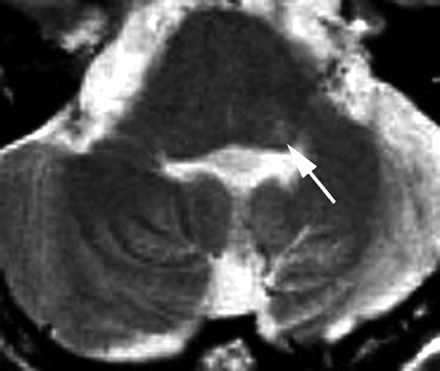
MR image of a 53-year-old man obtained 3 years and 1 month after surgery. A 3-mm-thick FSE T2-weighted image obtained at the level of the inferior cerebellar peduncle shows a tiny area of hyperintensity in the left dorsal portion in the brain stem (arrow).
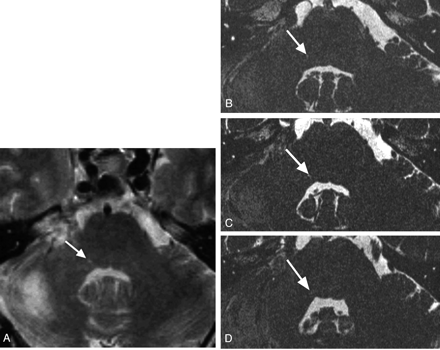
MR images of a 52-year-old man obtained 1 year and 3 months after surgery.
A, A 3-mm-thick FSE T2-weighted image obtained at the level of the middle cerebellar peduncle shows a focal hyperintensity at the right lateral angle of the fourth ventricle in the brain stem (arrow).
B–D, Alternate CISS MR images show that the hyperintensity is contiguous (arrows). The lower end of the hyperintense area is located more anteriorly and deeper on the fourth ventricle floor, and the upper end is more posteromedial.
Clinical and MR features of the 6 patients with vestibular schwannoma showing a focal hyperintensity in the dorsal brain stem
Hyperintensity was also seen in the same location on preoperative MR images in these 6 patients on both T2-weighted spin-echo and CISS images (Fig 3).
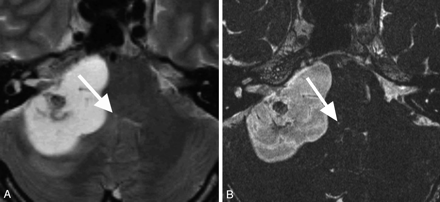
MR images of the patient in Fig 2 obtained before surgery.
A, FSE T2-weighted MR image shows a large cystic schwannoma. The fourth ventricle is compressed by the tumor, and the tiny area of hyperintensity can be seen at the same site (arrow).
B, A CISS MR image shows the hyperintense area more clearly (arrow).
Available preoperative MR images were also reviewed in the remaining 17 cases of vestibular schwannoma without focal hyperintensity in the brain stem. The dimensions of the largest vestibular schwannoma were 48 × 30 × 30 mm in these cases. Except for 6 vestibular schwannomas with the maximum diameter of less than 20 mm, the middle cerebellar peduncle was deformed and the vestibular nerve was displaced variably by schwannoma.
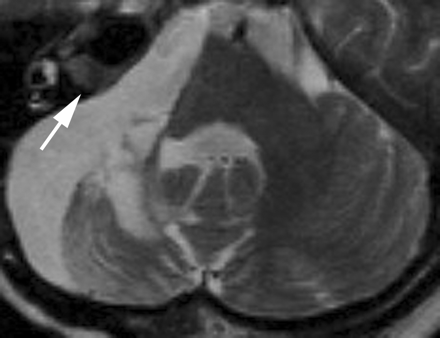
Such a hyperintense lesion was not found in the remaining patients with other kinds of cerebellopontine angle tumor or in patients in the control group. The largest meningioma (53 × 38 × 45 mm) with hydrocephalus, the largest tumor in this study, extended into the whole internal auditory canal and displaced the brain stem markedly. The facial and vestibular nerve complex could not be identified on preoperative MR images. After subtotal removal of the tumor, the distal anterior inferior cerebellar artery (AICA) syndrome developed (Fig 4). Even in this patient, postoperative MR images showed no hyperintensity in the brain stem.
MR image, obtained 13 months after surgery, of a 46-year-old woman with a large cerebellopontine angle meningioma. A 3-mm-thick FSE T2-weighted image obtained at the level of the middle cerebellar peduncle shows atrophic change of the right middle cerebellar peduncle and right cerebellar hemisphere, with a dilated fourth ventricle secondary to infarction in the right anterior inferior cerebellar artery distribution (distal AICA syndrome). Most of the meningioma was removed, but a residual tumor is seen in the enlarged right internal auditory canal as an isointensity lesion (arrow). No focal hyperintensity lesion is observed in the dorsal brain stem.
Five of the remaining 6 cases of meningioma (17–37 mm in the maximal diameter) extended into the internal auditory canal completely (3 meningiomas) or partially (2 meningiomas), and 6 meningiomas showed displacement of the vestibular nerve in the cerebellopontine angle cistern and compression of the middle cerebellar peduncle variably. No tiny hyperintensity was observed in the brain stem in these patients with meningioma. Three epidermoid cysts (32–37 mm in the maximal diameter) extended into the internal acoustic canal and encased the facial and vestibular nerves in the tumor. The middle cerebellar peduncle was displaced mildly in 2 patients. In a patient with glioma, the vestibular nerve in the cerebellopontine angle cistern was markedly displaced by the tumor (37 × 20 × 26 mm). The internal auditory canal was destroyed and was fully occupied by chordoma (44 × 27 × 37 mm) in 1 patient. No hyperintensity was demonstrated in the brain stem on pre- and postoperative MR images in these patients.
Discussion
Wallerian degeneration has been clearly demonstrated on MR imaging of the living human brain as a nonenhancing thin band of high T2 signal intensity contiguous with various primary lesions and conforming to the known anatomic pathway of a white matter tract, the corticospinal tract, or the pyramidal tract.4,5 The term wallerian degeneration remains in use for any form of antegrade degeneration of axons and their myelin sheaths, whether secondary to injury to the axon proximally or after the death of its cell body.4 Neuronal damage caused by a focal brain lesion influences function and morphology in the intact regions remote from the primary injury. MR imaging can also show secondary retrograde degeneration—for example, the secondary degeneration of the thalamus as the end of the thalamocortical or corticothalamic pathway after infarction of the middle cerebral artery distribution, seen as a hyperintense lesion on T2-weighted spin-echo images.6 In some specific groups of neurons, degeneration can be transferred to both afferent and efferent neurons via synapses, and this transfer is known as transneuronal degeneration.7 Recently, transneuronal degeneration of the limbic system has been demonstrated on MR imaging.7
In the present study, 6 patients with a relatively large vestibular schwannoma (>2 cm in the maximal diameter) showed a thin hyperintense area in the dorsal pons. The location and nonenhancing hyperintensity of the region suggest the degeneration of the vestibular nuclear complex. The vestibular nuclear complex consists of 4 separate nuclei, each receiving afferent information from several different sources.8 The medial and superior vestibular nuclei receive information directly from the cristae of the semicircular canals. In addition, the vestibular nuclei receive sensory input from the otolith organs; from proprioceptors, particularly those in the neck; as well as visual information. The cerebellum is also a major source of afferent information, providing significant contributions to the superior, lateral, and medial vestibular nuclei, and overall has important inhibitory control over vestibulo-oculomotor function.8
The normal vestibular nuclear complex is difficult to visualize directly on MR imaging.1-3 The most informative transverse brain stem image is at the level of the pontomedullary junction. The superior, medial, and lateral vestibular nuclei can be localized to the most lateral aspect of the floor of the fourth ventricle medial to the cochlear nuclei.8,9 The location of the hyperintense area demonstrated on T2-weighted spin-echo or CISS images in patients with relatively large vestibular schwannoma was at the lateral angle of the fourth ventricle floor dorsal to the inferior cerebellar peduncle (Figs 1 and 2) and was compatible with that of the vestibular nuclear complex.1-3,9,10 In addition, such a hyperintense area was not seen in patients with other kinds of cerebellopontine angle tumors. Histopathologic changes including wallerian degeneration, are observed in the vestibular nerve compressed by the tumor in patients with relatively large vestibular schwannomas.11 Our results suggest continuation of the wallerian degeneration to the nuclear complex level in patients with vestibular schwannoma lesions larger than 2 cm.
Distal AICA syndrome is one of the postsurgical complications in patients with vestibular schwannoma. In this syndrome, a variable area of hyperintensity is seen in the ipsilateral middle and superior cerebellar peduncles on postoperative MR images.12 The territory consistently supplied by the AICA and its tributaries includes the middle and parts of the superior cerebellar peduncles, the inferior portion of the cerebellum, the contents of the internal auditory canal, and the flocculus. The AICA gives a variable contribution to the lateral portion of the pons and upper lateral medulla.13 The larger tumor size (average, 3.8 cm; range, 2.0–5.5 cm in diameter) causes loss of the distal ramification of the AICA, resulting in focal cerebellar peduncle infarction.
The incidence of distal AICA infarction is 13% for tumors larger than 3 cm.12 The incidence of a tiny area of hyperintensity observed in our patients was 14.6%, and this hyperintense area was seen in patients with relatively large vestibular schwannomas (>2 cm in maximal diameter). However, the focal area of hyperintensity was not postoperative injury, such as the distal AICA syndrome, because the focal hyperintensity was also demonstrated on preoperative MR images. More important, the hyperintense area at the lateral angle of the fourth ventricle was identical in location and size on preoperative MR images of all these patients.
The hyperintensity could not be edema because the hyperintensity remained for a long period after surgery, 5 months or longer. In reviewing the preoperative MR images, we found the focal hyperintensity only in the same patients showing the hyperintensity postoperatively. No preoperative hyperintensity at the site in the brain stem disappeared postoperatively. If the hyperintensity is a perivascular space, it may not be seen preoperatively because the dilated perivascular space could be easily compressed by a large tumor.
We could not conclude that the hyperintensity is specific to the vestibular schwannoma. However, facial nerve schwannoma rarely presents as a cerebellopontine angle tumor.14 The 7 meningiomas included in this study compressed the vestibular nerve, 4 meningiomas involved the internal auditory canal completely, but the hyperintensity was not observed in the dorsal brain stem on either T2-weighted FSE or CISS images in these patients. Tumor extension into the internal auditory canal from the posterior fossa and/or mechanical compression of the vestibular nerve in the cerebellopontine angle cistern may not be a cause of the focal hyperintensity in the dorsal brain stem.
Correct preoperative diagnosis of a large cerebellopontine angle tumor is important because a different surgical approach is selected. The retrosigmoid suboccipital approach is recommended as most appropriate for a cerebellopontine angle meningioma, to remove it completely and to avoid the risk of recurrence,15 but the enlarged translabyrinthine approach should be considered for vestibular schwannoma, even one 3 cm or larger.16
Conclusion
The tiny area of hyperintensity at the lateral angle of the fourth ventricle posterior to the inferior cerebellar peduncle in patients with vestibular schwannoma greater than 2 cm is most likely due to degeneration of the vestibular nuclear complex. Therefore, the diagnosis of vestibular schwannoma should be suggested if a tiny hyperintense area is seen on preoperative MR images in patients with large cerebellopontine angle tumors and should not be confused with a small infarction or a postsurgical complication on postoperative MR images.
References
- Received May 9, 2005.
- Accepted after revision October 31, 2005.
- Copyright © American Society of Neuroradiology