Abstract
BACKGROUND: The MERCI (Mechanical Embolus Removal in Cerebral Ischemia) trial reported efficacy of the Merci Retriever for opening intracranial vessels in patients ineligible for intravenous (IV) tissue plasminogen activator (tPA). Patients who receive IV tPA but do not recanalize may also benefit from thrombectomy, but the revascularization efficacy and safety of this strategy has not been reported.
METHODS: Multi MERCI is an ongoing international, multicenter, prospective, single-arm trial of patients with large vessel stroke treated within 8 hours of symptom onset. Patients were enrolled who had received IV tPA but did not recanalize or who were ineligible for IV tPA. Primary outcome was vascular recanalization (Thrombolysis in Myocardial Infarction [TIMI] score II/III) and safety.
RESULTS: One hundred eleven patients received the thrombectomy procedure. Mean age ± SD was 66.2 ± 17.0 years, and baseline National Institutes of Health Stroke Scale (NIHSS) score was 19 ± 6.3. Thirty patients (27%) received IV tPA before intervention. Treatment with the Retriever alone resulted in successful recanalization in 60 of 111 (54%) treatable vessels and in 77 of 111 (69%) after adjunctive therapy (IA tPA, mechanical). Symptomatic intracranial hemorrhage (ICH) occurred in 10 of 111 (9.0%). Clinically significant procedural complications occurred in 5 of 111 (4.5%) patients. The symptomatic ICH rate was 2 of 30 (6.7%) in patients pretreated with IV tPA and 8 of 81 (9.9%) in those without (P > .99).
CONCLUSIONS: Mechanical thrombectomy after IV tPA seems as safe as mechanical thrombectomy alone. Mechanical thrombectomy with both first- and second-generation Merci devices is efficacious in opening intracranial vessels during acute ischemic stroke in patients who are either ineligible for IV fibrinolytic therapy or have failed IV fibrinolytic therapy.
The chief goal in treating acute ischemic stroke is to restore cerebral blood flow as rapidly and safely as possible. Intravenous (IV) tissue plasminogen activator (tPA) achieves early recanalization in only 30%–50% of patients, with even lower recanalization rates in proximal large vessel occlusions (middle cerebral, basilar artery, and carotid terminus),1,2 and reocclusion of the vascular segment occurs frequently.3,4 Because this size of vessel is navigable via catheter, endovascular techniques such as intraarterial (IA) thrombolysis5 or mechanical thrombectomy6,7 are feasible and may produce better vascular patency and improve clinical outcomes compared with IV tPA. As a practical algorithm, combining IV tPA initial treatment followed by IA tPA for patients whose vessels do not open has shown promise.8 Combining IV tPA pretreatment with mechanical thrombectomy may also improve the rate of recanalization, but the safety of this approach has not been previously documented. The Multi MERCI trial was designed in part to document safety of this IV tPA and mechanical thrombectomy combination therapy.
Methods
Patients and Techniques
Multi MERCI is an ongoing international, multicenter, single-arm trial that uses a family of thrombectomy devices (Merci Retriever X5, X6, and L5 models [Concentric Medical, Inc, Mountain View, Calif]) to restore cerebral perfusion within 8 hours of stroke symptom onset. The trial had 3 broad aims: 1) to gain greater experience with the first-generation Merci Retriever devices (X5 and X6) in patients ineligible for tPA, supplementing the data obtained in the MERCI trial6; 2) to explore the safety and technical efficacy of using the Merci Retriever in patients treated with IV tPA who failed to recanalize promptly; and 3) to obtain safety and technical efficacy data on a second-generation thrombectomy device (L5) once these became available for trial investigation. The X5 and X6 models were cleared for clinical use in August 2004, and the L5 model was used under an FDA-approved Investigational Device Exemption (IDE) as part of this trial. The trial enrolled patients at 14 sites, including 2 Canadian sites (shown in the appendix) and was approved by each local institutional review board. The study was supervised by an independent data safety monitoring board (DSMB). The results reported here are an interim analysis of the safety of combining IV tPA with mechanical thrombectomy.
Patient eligibility in the IV tPA ineligible arm of Multi MERCI was the same as the MERCI trial.6 Eligibility in the IV tPA treated arm was the same as in the IV tPA ineligible arm except that patients who had received tPA within 3 hours of onset under FDA-labeled indications could be enrolled if tPA failed to open the intracranial large vessel as proven by conventional angiography. In particular, patients were eligible who met all of the following criteria: age ≥18 years, signs and symptoms of acute stroke, National Institutes of Health Stroke Scale (NIHSS) score ≥8, and stroke symptom duration under 8 hours. After cerebral angiography, eligible patients had to have occlusion of a treatable vessel. Treatable vessels were defined as the intracranial vertebral artery, basilar artery, intracranial carotid artery (ICA), ICA terminal bifurcation (ICA-T), or the middle cerebral artery (MCA) first division (M1) or second division (M2). The patient was defined as enrolled once the balloon guide catheter was placed in the vasculature.
Patients were ineligible for the study if any of the following were true: informed consent was not obtained (and approval for waiver of explicit consent for emergency circumstances had not been obtained at the study site), current pregnancy, serum glucose <50 mg/dL, excessive tortuosity of cervical vessels precluding device delivery/deployment, known hemorrhagic diathesis, known coagulation factor deficiency, oral anticoagulation treatment with international normalized ratio >3.0, use of heparin within 48 hours, a partial thromboplastin time greater than 2 times normal, platelet count <30,000/μL, history of severe allergy to contrast media, sustained systolic blood pressure >185 mm Hg or diastolic blood pressure >110 mm Hg despite treatment, CT scan revealing significant mass effect with midline shift, greater than 50% stenosis of the artery proximal to the target vessel, or life expectancy under 3 months.
Embolectomy Procedure
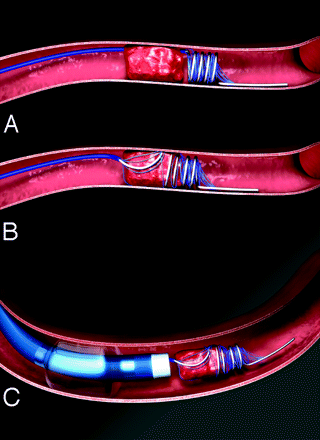
All patients underwent conventional cerebral angiography. Investigators were instructed to perform 4-vessel cerebral angiography before determining eligibility for the trial. After enrollment, patients were given intravenous heparin during the procedure. Upon inclusion of the next generation Retriever, the Merci Retriever L5, investigators were instructed to first attempt embolectomy with the L5 device shown in Fig 1. Subsequent passes could be made with either the L5, X5, or X6 devices. The Merci Retriever L5 differs from the previously cleared X5 and X6 devices by the inclusion of a system of arcading filaments attached to a nontapering helical nitinol coil. The Merci Retrieval System consists of a balloon guide catheter (8F or 9F), the Merci Retriever (L5, X5, X6), and a Merci Microcatheter (MC 14X or MC 18L). The balloon guide catheter was inserted transfemorally and placed in the proximal internal carotid artery (for anterior circulation stroke) or the subclavian or vertebral artery (for posterior circulation stroke). With the balloon of the guide catheter deflated, a 0.014-inch guidewire was advanced through the clot within the occluded intracranial vessel. The microcatheter was then advanced over this wire through the clot, and the guidewire was exchanged for the embolectomy device. The device was advanced so that up to 4 of the distal loops of the helix deployed distal to the clot. The microcatheter and the device were then pulled back to fully engage the clot, then the proximal loops of the device were deployed by further retraction of the microcatheter. The balloon of the balloon guide catheter was then inflated to arrest flow within the proximal arterial segment (to prevent distal embolization), and the microcatheter and embolectomy device were gently withdrawn into the body of the guide catheter while aspirating with a syringe. The balloon was then deflated and the clot was retrieved. Up to 6 Retriever passes within the vessel were allowed. If flow was restored with 6 or fewer passes of the device, successful recanalization was attributed to the device. Successful revascularization was defined as achieving Thrombolysis in Myocardial Infarction9 (TIMI) II or III flow in all treatable vessels. Successful recanalization for the MCA required all M1 and M2 segments to be at least TIMI II; for ICA-T lesions, the ICA, M1, and M2 branches needed to be at least TIMI II, and for the posterior circulation, both the vertebral and basilar arteries needed to be at least TIMI II to be considered recanalized. TIMI scoring of angiography was done by the local investigator who was not blinded to clinical outcome. If the treatable vessel was not opened to at least TIMI II flow with a maximum of 6 passes with the device, it was considered a treatment failure for the device. Intraarterial fibrinolytics (up to 24 mg tPA) were allowed in cases of treatment failure with the device, or to treat distal embolus not accessible to the device after successful proximal embolectomy. Use of glycoprotein (GP) IIb/IIIa antagonists and alternate mechanical thrombectomy procedures were prohibited. Aspirin but not intravenous heparin was allowed in the first 24 hours after the procedure.
Illustration of the L5 thrombectomy device removing thrombus. The L5 device is a helix of flexible nitinol wire with an arcade of filaments secured to the loops of the helix. This differs from the X5 and X6 Merci Retrievers by having filaments and no taper to the coils. Within 8 hours of acute ischemic stroke, the balloon guide catheter is placed via femoral artery into the proximal internal carotid or vertebral artery. The blue microcatheter is advanced through the balloon guide catheter and placed through the occlusion using a microguidewire. The guidewire is then exchanged for the Retriever, which is advanced distal to the clot and several loops are deployed (A). The device is further deployed so as to fully ensnare the clot (B). Then, the proximal balloon of the guide is inflated to prevent distal embolization, some torquing maneuvers are applied, and the microcatheter and Retriever are withdrawn together to retrieve the clot (C).
Clinical Variables and Measurement of Outcome
Patient demographics, medical history, vital signs, and routine laboratory values were documented on standardized clinical report forms. The National Institutes of Health Stroke Scale (NIHSS) and modified Rankin scores (mRS) were obtained at baseline and at 30 and 90 days. CT brain imaging was performed at baseline, at 24 hours, and at any time there was a decline in patient neurologic status. Symptomatic intracranial hemorrhage was defined as a ≥4-point decline in the NIHSS score within 24 hours with any blood products identified on head CT scan at 24 hours (petechial bleeding, hematoma, or subarachnoid hemorrhage), or any intracranial hemorrhage in which no further NIHSS scores were available beyond baseline and the patient died. All CT scans at 24 hours were reviewed at a central core lab. All hemorrhages were reviewed by the DSMB and adjudicated as to whether they were related to the procedure. Asymptomatic hemorrhage was defined as evidence of any blood on the CT scans at 24 hours or MR imaging scan with no more than a 3-point decline in the NIHSS score.
Primary outcomes were the rate of vascular recanalization and the observed rate of procedure-related complications. Recanalization was defined as TIMI grades II and III flow assessed immediately after treatment with the device. Procedure-related adverse events were defined as vascular perforation, intramural arterial dissection, or embolization of a previously uninvolved territory, symptomatic hemorrhage adjudicated as procedure-related, and access site complications requiring surgery or transfusion. Clinically significant procedural complications were defined as a procedure complication with decline in NIHSS of ≥4 points or death, groin complication requiring surgery, or blood transfusion. Secondary outcomes included clinical outcome, as measured by the mRS at 90 days, 90-day mortality, and safety dichotomized by use or no use of IV tPA. Good neurologic outcome was defined as mRS ≤ 2.
Statistical Analysis
Primary outcomes are reported based on patients who had the Retriever deployed. Clinical and demographic variables were tested as predictors of mortality and any type of intracranial hemorrhage. These variables included baseline NIHSS score, age, sex, time to treatment, site of vascular occlusion, revascularization, number of attempts to remove clot, duration of procedure, usage and dose of IV and/or IA tPA, investigational site, device model used, and protocol violations. Statistical tests used to determine the significance of differences in variables are listed in the data tables and within the text where relevant. Logistic regression of predictors of good outcome included all variables with P < .20 from the univariate analysis, then considered variables in a forward and backward scheme to arrive at the best model. In the case of death, mRS were set to 6 and NIHSS score were set to 42. All analyses were performed with the use of SAS for Windows, version 8.2 (SAS Institute, Carey, NC).
Results
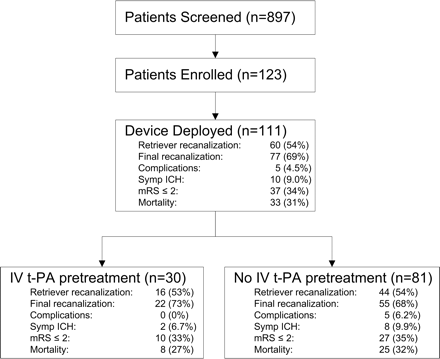
Part I of the Multi MERCI trial enrolled 123 patients and treated 111 patients with demographics and baseline characteristics shown in Table 1. Overall, 897 patients were screened, 123 were enrolled, and 111 patients had successful device deployment (Fig 2). Patient enrollment began January 20, 2004. The trial was placed on hold May 2, 2005 by the DSMB because of a question of safety regarding intracranial hemorrhages and protocol violations. Twelve patients did not have the device deployed for the following reasons: vessel tortuosity (n = 4), clot not penetrable with the microcatheter (n = 1), spontaneous recanalization (n = 2), distal clot migration to the M3 segment (n = 1), and presence of significant carotid stenosis (n = 4). All results are analyzed for the 111-patient cohort in which the device was successfully deployed.
Multi MERCI patient flow and primary outcomes. Recanalization is from device alone; final recanalization is after Retriever and any adjuvant therapy. Patients with symptomatic intracranial hemorrhage (ICH), and procedural complications may overlap.
Baseline characteristics of treated subjects
All patients underwent conventional angiography and were enrolled in the trial based on complete occlusion of the intracranial vessels shown in Table 1. There were no significant differences in age, baseline NIHSS score, and location of vascular occlusion between those receiving IV tPA and those not treated with IV tPA.
Thirty patients (27%) received IV tPA before angiography (Table 2). Nineteen of 30 patients received lower dose tPA (0.6 mg/kg) and 11 received standard dose tPA (0.9 mg/kg). In the 111-patient cohort, revascularization of the occluded intracranial vessel was achieved with the Retriever alone in 60 patients (54%) as shown in Table 3. Additional IA therapy with tPA was used in 43 cases (39%) where the Retriever failed to open the vessel or in cases where the Retriever opened the target vessel but a more distal vascular occlusion beyond reach of the device was seen. Adjunctive IA tPA was used in 13 patients who had received pre-Retriever IV tPA and 30 patients who had received no IV tPA. After all adjunctive therapies, recanalization was achieved in 77 patients (69%). Recanalization rates in patients receiving IV tPA and those not receiving IV tPA were similar, as were clinical outcomes, mortality, and rates of intracranial or extracranial bleeding as shown in Table 3. Procedure-related serious adverse events occurred in 11 patients (9.9%) but clinically relevant procedural complications occurred in only 5 patients (4.5%). No patient receiving IV tPA had a clinically significant procedural complication.
Fibrinolytic dosing
Primary results
Among cases in which the L5 device was the first device deployed, primary recanalization was achieved in 45 of 78 cases (58%) compared with recanalization in 15 of 33 (46%) of cases in which older generation X5/X6 devices were the first device deployed (P = .298). After adjunctive IA tPA, recanalization was achieved in 56 of 78 patients (72%) in whom the L5 device was used compared with 21 of 33 (64%) patients treated with the X5 or X6 only. Rates of any type of hemorrhage were similar between devices.
Major protocol violations occurred in 38 patients (34%). These included use of abciximab (n = 9), simultaneous deployment of 2 Retriever devices (n = 3), use of IA lytics before or between passes of the Retriever device (n = 13), use of other mechanical devices (n = 17), administration of more than 24 mg of tPA IA (n = 4), and use of IA tPA beyond 6 hours of stroke symptom onset (n = 9), treatment beyond 8 hours of symptom onset (n = 2), dose of IA tPA exceeding 24 mg (n = 4), and exceeding 6 passes with the Retriever (n = 1). Significantly more major protocol violations occurred in patients with at least one serious adverse event (Table 4). Major protocol violations did not significantly affect the symptomatic intracranial hemorrhage rate, but there was a trend toward more asymptomatic subarachnoid hemorrhages and higher mortality in patients with protocol violations. Multivariate analysis was performed using all demographic variables and procedure-related variables listed in the methods in an exploratory analysis of any type of hemorrhage and mortality. Use of IV tPA, IA tPA, or combination IV/IA tPA was not predictive of death or any type of hemorrhage. However, use of abciximab was predictive of asymptomatic subarachnoid hemorrhage (odds ratio [OR], 19.2; 95% confidence interval [95% CI], 3.68 to >100), and use of IA tPA before or between passes of the device was associated with subarachnoid hemorrhage (OR, 7.08; 95% CI, 1.36 to 37).
Major protocol violations
Discussion
The Multi MERCI Part I results show that combining IV tPA with endovascular thrombectomy does not substantially increase the risk of hemorrhage or serious adverse events. Compared with the MERCI trial, which enrolled patients who were ineligible for IV tPA, recanalization was achieved more commonly in Multi MERCI, even in patients not treated with IV tPA. Use of a second-generation device (L5) or increased operator experience are potential explanations for this increased recanalization rate.
These results are important to better understand the safety of the bridging strategy of combining IV tPA with subsequent endovascular intervention. This was first tested in the EMS bridging trial10 and later in IMS-I,8 where IV therapy was followed by IA tPA for persistent large-vessel intracranial occlusion. In the IMS I trial, successful recanalization of all intracranial vessels was achieved in 56% of patients by using a different but comparable measure of recanalization. This came at the expense of a symptomatic intracranial hemorrhage rate of 6%, groin hemorrhages rate of 3.8%, and intracranial vascular perforation rate of 3.8%.8 Multi MERCI used a similar design but allowed a higher pretreatment dose of IV tPA (0.9 mg/kg in 11 cases) and, within the cohort pretreated with IV lytic, showed a recanalization rate of 53% with the device alone and 73% after any adjunctive therapy, a symptomatic ICH rate of 6.7%, no groin hemorrhages, and a vascular perforation rate of 0%. Thus, combining IV tPA and endovascular thrombectomy seems to be comparable in safety to the combination of IV and IA fibrinolytics without thrombectomy. Comparison of efficacy between these trials is problematic, however, because both trials are single-armed, and there are differences in baseline patient characteristics including age, vessels treated, and stroke severity.
Multi MERCI also tested a newer generation device (L5) designed to allow better ensnarement of the intravascular clot. In this interim analysis, use of the L5 device was associated with an absolute 12% higher rate of recanalization compared with the first generation devices. This device will be further tested in Part II of Multi MERCI. Compared with the MERCI trial,6 Multi MERCI enrolled patients of similar age (mean age, 66 versus 67 years) and similar stroke severity (mean NIHSS scores, 19 versus 19). Trends in safety and efficacy are favorable when results of Multi MERCI are compared with those of MERCI.6 Specifically, compared with MERCI, Multi MERCI reports a higher Retriever alone recanalization rate (54% versus 48%), higher final recanalization rate (69% versus 60%), better 90-day clinical outcome (34% versus 28% mRS ≤ 2), fewer clinically significant procedural complications (4.5% versus 7.1%), and lower 90-day mortality (31% versus 44%). Because age and baseline stroke severity are similar between these 2 trials, the better neurologic outcome and lower mortality is probably explained by the better recanalization rates seen in Multi MERCI. These trends will be analyzed for significance upon completion of Multi MERCI Part II.
During the course of the Multi MERCI trial, several patients were not treated strictly within the protocol. In particular, some investigators used GP IIb/IIIa antagonists, deployed 2 Retrievers simultaneously, administered IA tPA before or between passes with the device, or used other mechanical thrombectomy devices. Exploratory analysis revealed concern particularly for use of IV abciximab and use of IA tPA before or between passes. Because the numbers of both treated patients and specific protocol violations are small, these results are not conclusive. However, under advice of the independent DSMB, the trial will continue in part II with more strict attention to preventing protocol violations. The trial continues with an executive review board to review any future major protocol violations and has the authority to close the specific site to further patient enrollment.
This trial has several limitations. Use of IV tPA was not randomly distributed, and all patients who entered the trial were either patients who did not receive IV tPA or patients who received IV tPA and specifically did not recanalize. Therefore, it would not be correct to conclude that IV tPA had no effect on recanalization in light of our observation that recanalization rates between patients pretreated or not pretreated with IV tPA are similar. In addition, the dosage of IV tPA was not standardized but followed 2 distinct doses at the discretion of the investigator. Therefore, the post hoc P values shown in Tables 3 and 4 were calculated only to explore potential safety concerns and should not be considered predictive of results from a randomized trial. Based on the proportions of adverse events reported herein, and considering the numbers of patients actually studied, this study is insufficiently powered for us to be firm in our conclusions.
Conclusions
Use of IV tPA before conventional angiography and attempted thrombectomy with the Merci Retriever does not seem to increase the incidence of intracranial hemorrhage compared with patients not treated with IV tPA.
Appendix
International Principal Investigator:
Wade S. Smith, MD, PhD, University of California, San Francisco.
Data Safety Monitoring Board:
Chair: Gene Sung, MD, MPH, University of Southern California; Biostatistician: Phil Hormel, MS; Members: Tim W. Malisch, MD, Alexian Brothers Medical Center; Steven Rudolph, MD, Maimonedes Medical Center, Arun Amar, MD, Stanford University.
Imaging Core Lab:
Paul Kim, MD, University of Southern California.
Writing Committee:
Ronald Budzik, MD, Gary Duckwiler, MD, Donald Frei MD, Y. Pierre Gobin, MD, Thomas Grobelny, MD, Randall T. Higashida, Frank Hellinger, MD, Dan Huddle, MD, Chelsea Kidwell, MD, Walter Koroshetz, MD, David S. Liebeskind, MD, Helmi L. Lutsep, MD, Michael Marks, MD, Gary Nesbit, MD, Marilyn M. Rymer, MD, Jeffrey Saver, MD, Isaac E. Silverman, MD, Don Smith, MD, Wade S. Smith, MD, PhD, Sidney Starkman, MD, Gene Sung, MD, MPH.
Site Principal Investigator (PI), Coinvestigators, and Study Coordinators in order of enrollment (N):
Saint Luke’s Hospital: (41) PI: Thomas Grobelny, MD, Naveed Akhtar, MD, Steven Arkin, MD, Irene Bettinger, MD, Marilyn Rymer, MD, Charles Weinstein, MD, Michael Schwartzman, MD, Christine Boutwell, MD, Barbara Gruenenfelder, RN, Annette Allen, RN. Riverside Methodist Hospital: (16) PI: Ronald Budzik, MD, Geoffrey Eubank, MD, Erik Arce, MD, Jim Fulop, MD, John Lippert, MD, Tom Davis, MD, J. Kevin McGraw, MD, Peter Pema, MD, Paula Meyers, RN. Oregon Stroke Center: (12) PI: Helmi Lutsep, MD, Stanley Barnwell, MD, Wayne Clark, MD, Ted Lowenkopf, MD, Elizabeth North, MD, Joseph Quinn, MD, Robert Egan, MD, Todd Kuether, MD, John Roll, MD, Gary Nesbit, MD, Christopher Zylak, MD, Barbara Dugan, RN. The Stroke Center at Hartford Hospital: (8) PI: Isaac Silverman, MD, Stephen Ohki, MD, Gary Speigel, MD, Martha Ahlquist, LPN, CCRP, Dawn Beland, MSN. Florida Hospital Neuroscience Institute: (7) PI: Frank Hellinger, MD, Susan Mitchell, RN. Swedish (Denver) Medical Center: (6) Co-PIs: Don Frei, MD, Dan Huddle, MD, Don Smith, MD, Carol Greenwald, MD. Stanford University Medical Center: (1) PI: Michael Marks, MD, Huy Do, MD, Gregory Albers, MD, Amie Hsia, MD, Christine Wijamn, MD, Mary Marcellus, RN. University of California at Los Angeles Medical Center: (5) PI: Sidney Starkman, MD, Dennis Chute, MD, Gary Duckwiler, MD, Doojin Kim, MD, David S. Leibeskind, MD, Victor Marder, MD, Bruce Ovbiagele, MD, Venkatakrishna Rajajee, MD, Nerses Sanossian, MD, Jeffrey Saver, MD, Scott Selco, MD, Paul Vespa, MD, J. Pablo Villablanca, MD, Fernando Vinuela, MD, Reza Jahan, MD, Judy Guzy, RN. University of Calgary, Foothills Hospital (4): PI: Michael Hill, MD, Mark Hudon, MD, John Wong, MD, Will Morrish, MD, Karyn Fischer, RN. NY Presbyterian Hospital-Cornell: (4) PI: Alan Segal, MD, Ai-His Liu, MD, Igor Ougrets, MD, Howard Riina, MD, Y. Pierre Gobin, MD, Kimberly Salvaggio, NP. NY Presbyterian Hospital-Columbia: (3) PI: John Pile-Spellman, MD, Sean Lavine, MD, Sundeep Mangla, MD, Philip Meyers, MD, Leslie Schmidt, NP. Georgetown University: (3) PI: Vance Watson, MD, John DeSimone, MD, Manual Yepes, MD, Theresa Kowal, RN, Susan Sutten, MPH. University of Alberta, Edmonton: (2) PI: Ashfaq Shuaib, MD, Brenda Scwindt, RN. Baptist Memorial Clinical Research Center: PI: John Barr, MD, Paul Broadbent, MD, Soren A. Singer, MD, Stephen D. Morris, MD, Sanat Dixit, MD, Grace Miller.
Footnotes
Disclosure: The authors wish to disclose the following: Ronald F. Budzik, Jr., MD, is a consultant and speaker for Concentric Medical and received research support from the company; Gary Duckwiler, MD, Thomas Grobelny, MD, and Y. Pierre Gobin, MD, are stockholders in Concentric Medical; Gary M. Nesbit, MD, is on the Scientific Advisory Board of Concentric Medical; Randall T. Higashida, MD, and David S. Liebeskind, MD, are consultants for Concentric Medical; Chelsea S. Kidwell, MD, is principal investigator for the MR RESCUE trail for which Concentric Medical provided catheters and devices; Jeffrey L. Saver, MD, was lead investigator for the MR RESCUE NIH trial for which Concentric Medical provided devices, is a member of the Scientific Advisory Board and speaker’s bureau of Boehringer Ingelheim and received research support from the company, and was site investigator for the IMS 2 and IMS 3 NIH trial for which Genentech provided drugs; Wade S. Smith, MD, PhD, is a stockholder in Concentric Medical and received a research support from Boehringer-Ingelheim Pharmaceuticals; and Sidney Starkman, MD, received research support from Concentric Medical and Genentech.
See Appendix for affiliations.
References
- Received January 11, 2006.
- Accepted after revision March 3, 2006.
- Copyright © American Society of Neuroradiology