Abstract
Summary: Progressive multifocal leukoencephalopathy is a severe demyelinating disease of the central nervous system caused by a JC papovavirus infection. Prognosis is poor with fatal outcome within 1 year in 90% of patients. Our case report shows that diffusion tensor imaging can give early and specific information about disease status and extent in a 15-year-old girl with progressive multifocal leukoencephalopathy associated with congenital human immunodeficiency virus (HIV) infection.
Progressive multifocal leukoencephalopathy is a severe, demyelinating disease of the central nervous system due to JC papovavirus infection of the myelin-producing oligodendrocytes (1, 2). The name JC virus is derived from the initials of the index patient. Progressive multifocal leukoencephalopathy typically occurs in immunocompromised individuals, such as patients with congenital human immunodeficiency virus (HIV) infection or other conditions associated with impaired T-cell function (2, 3). In the course of the infection, extensive myelin breakdown results in white matter destruction. Neurologic symptoms are unspecific and include focal neurologic deficits and dementia (1, 2, 4). Without treatment, patients have a relentless downhill course. The disease is fatal within 1 year of diagnosis in 90% of patients (3). Diagnosis can be established by the detection of JC virus DNA in cerebrospinal fluid (5, 6).
Many reports have described MR imaging findings in progressive multifocal leukoencephalopathy, but only a few MR imaging abnormalities correlate with patient survival (1, 7, 8). Diffusion tensor imaging is a relatively new MR imaging technique, which allows quantification of the degree of tissue injury by measuring isotropic (apparent diffusion coefficient) and anisotropic (fractional anisotropy) diffusion within white matter (9). In addition, diffusion tensor imaging allows a differentiation of potentially reversible vasogenic and frequently irreversible cytotoxic brain tissue edema (9).
The goal of our report is to test whether changes in isotropic and anisotropic diffusion can be measured by diffusion tensor imaging within normal- and abnormal-appearing white matter on conventional MR imaging and whether these findings correlate with follow-up imaging in a 15-year-old girl with proven progressive multifocal leukoencephalopathy associated with congenital HIV infection.
Case Report
A 15-year-old girl with known perinatally acquired HIV infection was admitted to our hospital because of a rapidly deteriorating neurologic condition. The legal guardian had refused antiretroviral treatment. Neurologic examination revealed a left-dominant spastic tetraparesis, a left-sided facial nerve palsy, a left-sided hemianopsia, and aphasia.
Conventional T2-weighted fast spin-echo and pre- and postcontrast T1-weighted spin-echo imaging was followed by diffusion tensor imaging. Diffusion-weighted images and apparent diffusion coefficient (ADC) and fractional anisotropy maps were calculated. Regions of interest were manually outlined in multiple anatomic locations, including normal- and abnormal-appearing hemispheric white matter, white matter tracts, and central gray matter. All apparent diffusion coefficient and fractional anisotropy measurements were performed bilaterally. Measurements were compared with age-related normative data (10).
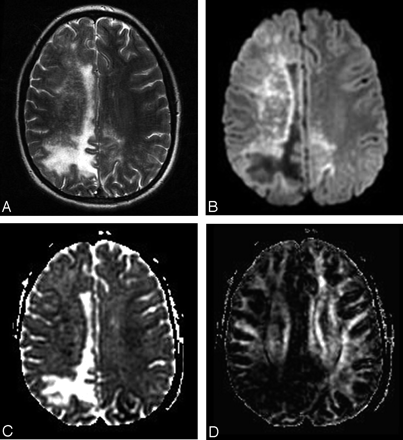
Conventional MR imaging revealed focal T2-hyperintense areas (right parietooccipital and precentral white matter) of demyelination without mass effect or contrast enhancement (Fig 1A). The lesions spared the subcortical U-fibers and overlying cortex. In addition, a discrete and diffuse patchy T2-hyperintensity was seen throughout the entire right hemisphere (Fig 1A). Diffusion-weighted images revealed matching areas of altered diffusion rates (Fig 1B). The right hemispheric punctuate lesions were more conspicuous on the diffusion-weighted images compared with T2-weighted images. On the ADC maps, the focal T2-hyperintense lesions were characterized by a center of increased diffusion rate indicating vasogenic edema or tissue necrosis (diffusion-weighted hypointense, ADC hyperintense) surrounded by a peripheral rim of reduced diffusion rate indicating cytotoxic edema (diffusion-weighted hyperintense, ADC hypointense) (Fig 1C). The diffuse punctuate T2-hyperintense right-hemispheric lesions matched multiple foci with reduced diffusion rates on the ADC maps (Fig 1C). Fractional anisotropy maps showed a markedly decreased anisotropic diffusion within the right-sided white matter (Fig 1D) as well as in the posterior limb of the internal capsula. The measured ADC and fractional anisotropy values are summarized in the Table. As shown in the Table, the ADC is within normal limits in normal-appearing white matter as well as within the internal capsule and the splenium of the corpus callosum. ADC is significantly increased within the center of the large T2-hyperintense right parieto-occipital lesion. The periphery of the focal lesion shows a significantly reduced ADC. The multiple punctuate lesions within the right hemisphere also show reduced ADC values. Fractional anisotropy is significantly decreased within the right occipital white matter, centrum semiovale, and parietal white matter. In addition, fractional anisotropy is also decreased within the right posterior limb of the internal capsule.
Initial MR imaging in a 15-year-old girl with perinatally acquired human immunodeficiency virus infection. T2-weighted fast spin-echo image shows hyperintense confluent area of demyelination (A) within the right parietooccipital white matter with extension into the right cingulate gyrus. The overlying cortex is spared. In addition, multiple punctuate T2-hyperintense lesions are seen throughout the entire right hemisphere. On diffusion tensor imaging, the parietooccipital T2-hyperintense lesion is centrally diffusion-weighted hypointense (B) and apparent diffusion coefficient (ADC) hyperintense (C), whereas the periphery of the lesion is diffusion-weighted hyperintense and ADC hypointense (rim of cytotoxic edema). On the fractional anisotropy map (D), the degree of anisotropy is markedly reduced. In addition, multiple small diffusion weighted–hyperintense, ADC-hypointense punctuate lesions are seen within the right hemisphere, compatible with multiple small foci of active inflammation with tissue injury.
Measured ADC and FA values in comparison with age-matched normative data
Diagnosis of progressive multifocal leukoencephalopathy was established by demonstrating JC virus DNA in cerebrospinal fluid by polymerase chain reaction testing. Antiretroviral therapy was started but had to be discontinued after 6 weeks because of rapid neurologic deterioration with impaired consciousness (Glasgow Coma Scale score declined from 13 to 8 within 24 hours). The child’s consciousness recovered on discontinuation of the antiretroviral treatment.
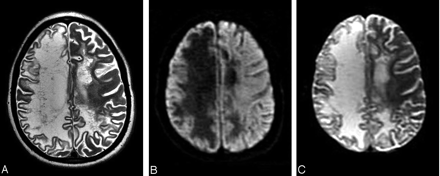
Follow-up MR imaging performed 13 months later showed a severe progression of demyelination within the entire right hemisphere as well as new areas of demyelination within the left hemisphere (Fig 2A). In addition, a significant global atrophy had developed. The overlying cortex remained intact. On isotropic diffusion-weighted images and corresponding ADC maps, no lesions with restricted diffusion were visible (Fig 2B, -C). The right internal capsule, which had shown a reduced anisotropic diffusion 13 months earlier, developed a T2-hyperintense signal intensity with increased diffusion on the ADC maps on follow-up imaging, indicating tissue loss.
Follow-up MR imaging 13 months later. Axial T2-weighted fast spin-echo image (A) shows an extensive T2-hyperintense destruction of the right hemispheric white matter with global atrophy. In addition, new focal T2-hyperintense lesions are seen within the left hemisphere. Diffusion-weighted imaging (B) shows a hypointensity with corresponding ADC hyperintensity (C) of the white matter within the right hemisphere as a result of extensive tissue injury and necrosis. No lesions with active inflammation (ADC hypointensity) are seen.
Neurologically, the child remained stable and is currently in a pediatric neuro-rehabilitation center.
Discussion
Many reports have described MR imaging characteristics of progressive multifocal leukoencephalopathy (1, 7, 8). However, few MR imaging findings correlate with patient outcome and survival. One large multicenter cohort study showed that except for mass effect, no MR imaging findings were predictive of the risk of death in patients with progressive multifocal leukoencephalopathy (1). The same authors, however, also mentioned that a mass effect was so infrequent and minimal that it was not a useful prognostic sign (1). The need for additional, more predictive diagnostic tests is obvious. These tests should be noninvasive, highly sensitive, and specific; should show pathology early in the course of the disease; and should be capable of quantifying the degree of tissue injury to guide and monitor treatment.
Diffusion-weighted imaging is a new MR imaging technique that has proven its value in evaluating brain tissue injury by quantifying isotropic water diffusion rates. This allows a differentiation of vasogenic and cytotoxic edema (9). By measuring the entire diffusion tensor, one can quantify the degree of anisotropic diffusion within the brain. The degree of anisotropic diffusion refers to the directionality of water diffusion in the brain, which is believed to be related to the degree of myelination of the white matter. Consequently, diffusion tensor imaging is a valuable imaging tool in diseases that affect the microstructural integrity and myelination of the brain as in progressive multifocal leukoencephalopathy.
Two recent articles have shown that the degree of isotropic and anisotropic diffusion is altered in normal-appearing white matter in adult patients with HIV infection (11, 12). They also showed that fractional anisotropy measurements were more prognostic of dementia than were ADC measures (12). In addition, 2 groups that studied adult patients with progressive multifocal leukoencephalopathy concluded that diffusion-weighted imaging can be used to monitor the degree of demyelination and tissue destruction (2, 13).
Our results show that diffusion tensor imaging reveals 2 kinds of lesions that most likely represent extremes of a continuous spectrum of tissue injury. Large focal lesions of T2 hyperintensity are characterized by a central area of increased diffusion (ADC hyperintense) surrounded by a small rim of decreased diffusion (ADC hypointense). This appearance indicates a concentric, centripetal process of tissue injury with central areas of completed tissue injury with necrosis surrounded by a progressing rim of active tissue injury with cytotoxic edema. In addition to the large focal lesions, diffusion-weighted/diffusion tensor imaging identified multiple small areas of decreased diffusion throughout the entire cerebral white matter. These lesions had varying diameters ranging between 2 and 5 mm. These lesions were significantly more conspicuous on the isotropic diffusion-weighted images than on the T2-weighted conventional MR images.
The findings on diffusion-weighted/diffusion tensor imaging parallel the findings that are described neuropathologically. The large lesions represent confluent areas of tissue loss, whereas the multiple small lesions resemble the moth-eaten appearance of active inflammation that is seen on histologic sections. Follow-up imaging 13 months later showed that the multiple punctuate lesions had evolved to a confluent hemispheric tissue injury with atrophy. In addition, on initial imaging, the anisotropic diffusion (fractional anisotropy) was reduced within the right internal capsula, whereas the isotropic diffusion (ADC) was within normal limits.
Conventional MR imaging did not show any correlating pathology. On follow-up imaging, the ADC value of the internal capsula was however increased, with concomitant T2-hyperintensity due to progressive injury of white matter tracts. In addition, fractional anisotropy measurements were reduced within areas of normal ADC values, which all evolved to tissue injury on follow-up imaging. These findings of a reduced fractional anisotropy value, while the ADC values remain less or not affected, parallel previous findings from Ragin et al (12) that fractional anisotropy measures are more prognostic than ADC measures in HIV-associated encephalopathy. The primary decrease in anisotropic diffusion also follows the pathologic–histologic sequence of progressive multifocal leukoencephalopathy, in which the myelin sheath of neurons and fiber tracts is primarily injured (fractional anisotropy decrease), followed by secondary diffuse cell loss (ADC increase).
In conclusion, diffusion tensor imaging can be a potential biomarker for the degree of tissue injury in progressive multifocal leukoencephalopathy because fractional anisotropy measurements can identify and quantify pathology along white matter tracts before conventional MR imaging or ADC maps show correlating abnormalities. These measurements could be used to guide and monitor treatment.
References
- Received July 14, 2004.
- Accepted after revision November 8, 2005.
- Copyright © American Society of Neuroradiology