Abstract
BACKGROUND AND PURPOSE: Movement-associated cortical changes have been detected at the earlier clinical stage multiple sclerosis. Our purpose was to assess whether different patterns of cortical recruitment are associated with the short-term evolution of definite multiple sclerosis (MS).
METHODS: We followed for 1 year a group of patients with clinically isolated syndromes (CISs) suggestive of MS and compared the baseline movement-associated patterns of cortical activations between those patients with and those without evolution to definite MS.
RESULTS: Those patients in whom MS did not evolve had more significant activations of several areas part of the “classic” motor network; those who went on to develop MS had more significant activations of several regions in the frontal, parietal, temporal, and occipital lobes.
CONCLUSION: In CIS patients, the extent of early cortical reorganization following tissue injury might be a factor associated with a different disease evolution.
In the past 15 years, several MR imaging markers have been investigated to improve our ability to predict disease evolution in patients with clinically isolated syndromes (CISs) suggestive of multiple sclerosis (MS) (1–3). Among these MR imaging markers, the extent of T2-visible lesions at disease onset has been shown to have a significant impact on the short-term clinical evolution to established MS and, albeit to a lesser extent, on the long-term accumulation of “fixed” disability (3).
Functional MR imaging (fMRI) is being widely used for the study of CNS functioning, in healthy individuals and in subjects with different neurologic conditions. Numerous fMRI studies have demonstrated that the human brain is capable of extensive cortical reorganization after acute or chronic CNS injuries (4, 5). Consistent with this notion, several fMRI studies have shown functional cortical changes in MS patients during the performance of motor, visual, and cognitive tasks (4). The correlation found between the extent of fMRI activations and the severity of brain and cord abnormalities suggests an adaptive role of cortical reorganization in limiting the functional consequences of tissue damage (4). In two previous fMRI studies (6, 7), we have demonstrated altered brain patterns of cortical activations during the performance of motor tasks of different complexity in patients at presentation with CIS suggestive of MS when compared with matched healthy volunteers.
The aim of this retrospective study was to investigate whether the nature and extent of movement-associated cortical recruitment differ in patients with CIS that either did or did not evolve to definite MS over a short-term follow-up period, which would suggest a possible influence of adaptive functional changes on the early course of the disease.
Methods
Patients
The 16 patients with CISs (8; 10 female, 6 male; mean age, 31.7 years; age range, 22–43 years) who underwent fMRI assessment at baseline (6, 7) were reassessed clinically and by conventional MR imaging after 3 (SD, 1 week) and 12 months (SD, 1 month). As reported elsewhere (6, 7), the presenting symptoms were optic neuritis, brain stem syndromes, and spinal cord syndromes in seven, three, and six patients, respectively (6, 7). None of the patients had ever had clinical symptoms involving the right upper limb. Oligoclonal bands were found in the CSF of 13 patients. The neurologic examinations at baseline month 3 and month 12 were performed by a single physician (M.A.R.) who was unaware of the fMRI and MR imaging results, and disability was measured by using the Expanded Disability Status Scale (EDSS). At baseline, 12 patients had EDSS scores of 0.0, and four had residual visual deficits, which resulted in EDSS scores of 1.0 (6, 7). During the study period, the occurrence of clinical relapses was noted by the same physician. At baseline, 15 sex- and age-matched healthy volunteers (nine women and six men; mean age, 33.6 years; age range, 21–45 years) served as control subjects for fMRI examination. Local ethics committee approval and written informed consent from all subjects were obtained before study initiation.
MR Imaging Acquisition
At study entry (within 3 months from the onset of clinical symptoms), each subject underwent conventional MR imaging of the brain (i.e., dual-echo and pre- and postcontrast T1-weighted sequences) and fMRI during the performance of a simple motor task with the dominant, clinically unaffected, right-upper limb. Further details about MR imaging examinations and the fMRI paradigm are reported elsewhere (6).
At follow-up, after careful repositioning of the patients, the following sequences of the brain were collected from all the patients: A) dual-echo turbo spin-echo (SE) (TR, 3300 ms; TE1, 16 ms; TE2, 98 ms; echo-train length, 5); B) T1-weighted conventional SE (TR, 768; TE, 14; NEX, 2) before and after the administration of 0.1 mmol/kg of gadolinium. For each sequence, 24 contiguous, 5-mm-thick, axial sections with a 256 × 256 matrix and a 250 × 250 mm2 FOV were acquired.
MR Imaging Analysis
At follow-up, new lesions were identified on the proton density-weighted and postcontrast T1-weighted images. The T2-weighted and the precontrast T1-weighted images were always used to increase confidence in lesion identification. Detailed information about single subjects’ fMRI analysis is reported elsewhere (6).
Statistical Analysis
The comparisons between the movement-associated patterns of cortical activation at disease onset between patients with and those without (clinical or MR imaging confirmed) disease evolution and between these two groups of patients and healthy volunteers were investigated by using a random-effect (t test for unpaired data) analysis (6, 7).
We report activations below a threshold of P < .05 corrected for multiple comparisons. To assess the correlation between fMRI and conventional MR imaging metrics, we used statistical parametric mapping (SPM) and linear regression analysis (6).
Results
Clinical and Conventional MR Imaging Findings
As previously reported (6, 7), all patients had paraclinical evidence of disease dissemination in space (8). On follow-up, 11 patients showed evidence of MR imaging disease dissemination in time (six at month 3 and five at month 12), thus fulfilling the criteria for a diagnosis of MS (8). MR imaging disease dissemination in time was associated with evidence of clinical disease dissemination in time over the study period in four patients. Only one of the patients who had a second clinical attack had residual neurologic deficits resulting in an EDSS of 2.0. Oligoclonal bands were present in 10 of the 11 patients with MS and in three of the remaining patients. Five of the 11 patients with MS were treated with immunomodulatory drugs at the time of the follow-up MR imaging. The median number of new lesions seen in these patients during the follow up was six (range, 1–27). The remaining five patients did not show any clinical or MR imaging evidence of temporal dissemination of the disease. Patients with MS and those without MS did not differ at baseline in terms of sex, age, motor performance, T2-weighted lesion load, normalized brain volume and whole brain n-acetylaspartate concentration (data not shown).
Functional MR Imaging
The contrasts between healthy volunteers and the two groups of patients showed no areas that were significantly more activated in healthy volunteers than in patients.
Patients with No Disease Evolution versus Healthy Volunteers
Compared with healthy volunteers, patients with no disease evolution had more significant activations of the contralateral primary somatomotor cortex (SMC) (SPM space coordinates: −22, −26, 62), contralateral supplementary motor area (SMA) (SPM space coordinates: −8, −2, 58) and ipsilateral parietal portion of the paracentral lobule (SPM space coordinates: 10, −46, 66).
Patients with Disease Evolution versus Healthy Volunteers
Compared with healthy volunteers, patients who developed MS had more significant activations of the superior frontal sulcus (SFS), bilaterally (SPM space coordinates: 26, −2, 54 and −20, −6, 46), the ipsilateral infraparietal sulcus (IPS) (SPM space coordinates: 32, −44, 68), the ipsilateral middle frontal gyrus (MFG) (SPM space coordinates: 38, −4, 54), the ipsilateral superior temporal gyrus (STG) (SPM space coordinates: 48, 12, −14), the contralateral fusiform gyrus (SPM space coordinates: −22, −52, −4), and the cuneus, bilaterally (SPM space coordinates: 22, −56, 14 and −18, −56, 4).
Patients with Disease Evolution versus Patients without Disease Evolution
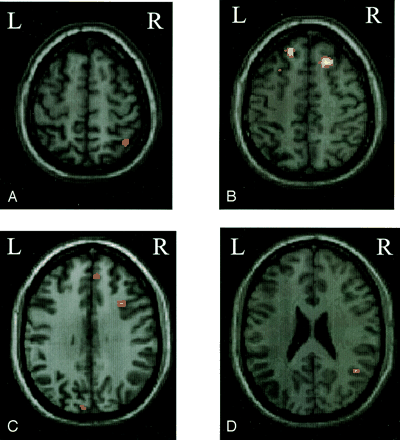
Compared with patients who went on to develop MS, those patients with no evolution had more significant activations of the contralateral primary SMC (SPM space coordinates: −20, −28, 64), contralateral SMA (SPM space coordinates: −4, 0, 60), ipsilateral parietal portion of the paracentral lobule (SPM space coordinates: 6, −46, 64), and ipsilateral cerebellar hemisphere (SPM space coordinates: 18, −58, −22) (Fig 1). On the contrary, compared with those patients without a disease evolution, patients who developed MS had more significant (bilateral) activations of the SFS (SPM space coordinates: 24, 26, 52 and −26, 18, 52), the superior frontal gyrus (SFG) (SPM space coordinates: 6, 50, 46 and −6, 36, 50), the IPS (SPM space coordinates: 40, −56, 60 and −30, −74, 36) (Fig 2), and the putamen (SPM space coordinates: 20, 2, 10 and −18, 2, 14). They also showed more significant activations of the ipsilateral MFG (SPM space coordinates: 34, 16, 34), STG (SPM space coordinates: 46, −54, 24) (Fig 2), and cuneus (SPM space coordinates: 22, −56, 14), as well as of the contralateral fusiform gyrus (SPM space coordinates: −22, −52, −4).
Color-coded SPMt maps superimposed on a high-spatial-resolution T1-weighted image showing relative cortical activations during a simple motor task with the dominant, functionally normal right hand in patients with clinically isolated syndromes suggestive of MS that did not evolve to definite MS compared with those who did (random effect analysis, between-group comparison, corrected P value < .05).
A, Ipsilateral paracentral lobule.
B, Contralateral primary somatomotor cortex and supplementary motor area.
C, Ipsilateral cerebellar hemisphere.
Color-coded SPMt maps superimposed on a high-spatial-resolution T1-weighted image showing relative cortical activations during a simple motor task with the dominant, functionally normal right hand in patients with clinically isolated syndromes that evolved to MS as compared with those who did not (random effect analysis, between-group comparison, corrected P value < .05).
A, Ipsilateral infraparietal sulcus.
B, Bilateral superior frontal sulcus.
C, Ipsilateral superior frontal gyrus and middle frontal gyrus and contralateral infraparietal sulcus.
D, Ipsilateral superior temporal gyrus.
In the two groups of patients, no correlation was found between the extent of fMRI activation and the numbers of total and new T2-visible lesions.
Discussion
Previous longitudinal MR imaging studies of patients with CIS have identified several factors associated to an increased risk of subsequent evolution to definite MS (1–3). Among them, the presence and extent of T2-visible lesions of the brain (1, 3), the severity of normal-appearing brain tissue involvement (2), and the degree of brain atrophy (3) are all likely to play a role. This study suggests that in patients at presentation with CIS suggestive of MS, different movement-associated pattern of cortical activations might be associated with a different short-term disease evolution.
Patients without disease evolution after 1 year had a baseline pattern of movement-associated activations, which involved almost exclusively the “classic” areas devoted to simple motor task performance, such as the primary SMC and the SMA. Conversely, patients with subsequent short-term disease evolution showed a widespread recruitment of several regions located in the frontal (SFG, SFS, MFG), parietal (IPS), temporal (STG), and occipital lobes. All of these regions are involved, in different stages and with different roles in movement performance. The activation of the frontal lobes might reflect an increased attentional demand (9). Basal ganglia have extensive connections to the motor and somatosensory cortices and are involved in motor programming, execution, and control (10). The parietal cortex—and specifically the IPS—is usually involved in the elaboration of somatosensory inputs (11) and in movement preparation and planning (12). Increased activity in the IPS has also been described in healthy subjects during complex finger-movement sequences (13). As a consequence, the increased recruitment of these widespread sensorimotor network in patients with CIS with subsequent short-term disease evolution might be the reflection of these patients’ perception of the experimental simple task as a complex or novel one, which, in turn, might be the result of a more severe structural tissue damage than that of patients who did not develop MS and showed a more focused cortical recruitment of areas strictly related to motor performance. Mental imagery is also likely to contribute to the observed pattern of cortical activations in patients with evolving CIS, because they had an increased recruitment of areas located in the temporal and occipital lobes, which are known to be part of the visual network (14).
Several fMRI studies of patients with MS have consistently shown that the extent of fMRI activations in MS is strongly correlated to the amount of macro- and microscopic brain and cord tissue damage (4). There is an increasing body of evidence showing that at least some of these changes occur early in the course of the disease (6, 7, 15). Therefore, it is likely that although not directly investigated in the present study, the extent, nature, and location of structural brain disease might have had a role in determining the patterns of cortical activations seen in these patients.
A variable that has been related to the movement-associated pattern of cortical activations in patients with established MS is the time elapsed between the clinical onset of the disease and fMRI acquisition (15); however, because only four patients of the current study had a clinical conversion of the disease and fMRI acquisition was very close to the first clinical attack, we cannot disentangle the influence, if any, of this variable on our results. Intersubject variability in the brain pattern of cortical activations might have been another factor influencing the results of between-group comparison. Nevertheless, the analysis of the single-subject results did not disclose any gross difference among the different patients.
Our observation that patients with evolving CIS tend to recruit a more widespread sensorimotor network than those without short-term disease evolution agrees with the finding of an abnormal cortical activation in patients at high risk of developing Alzheimer disease compared with healthy subjects (16, 17). This would suggest that, whereas increased recruitment of widespread sensorimotor network contributes in limiting the impact of structural damage during the course of MS (4), its early activation might be counterproductive, because it might result in early exhaustion of adaptive properties of the brain. This hypothesis is also supported by studies in stroke patients, in which a persistent overactivation and over-recruitment of a widespread cortical network has been related to an unfavorable clinical outcome (5).
Conclusion
Although these results should be considered as preliminary, because of the small sample of patients studied, this study suggests that in patients at presentation with CISs an altered pattern of cortical recruitment at disease onset might be among the factors related to a less favorable subsequent clinical evolution.
Footnotes
Supported by the Fondazione Italiana Sclerosi Multipla (grant number 2000/R/37).
References
- Received June 14, 2004.
- Accepted after revision September 17, 2004.
- Copyright © American Society of Neuroradiology