Abstract
Summary: We report a case of thoracic spine diskitis of unknown cause that had aggressive and destructive features on MR images. Results of two biopsies were unremarkable. The process began after sneezing, also produced extensive paraspinous enhancement, and resolved without antibiotic therapy both clinically and radiologically after four months. A discussion of case similarities to Reflex Sympathetic Dystrophy (RSD) in the extremities render this possibly the first reported imaging evidence of RSD in the axial skeleton.
Diskitis is an inflammatory process involving an intervertebral disk that may affect the adjacent vertebral bodies. In adults, diskitis is more commonly seen following spinal instrumentation, but there have been case reports of infectious etiologies, both bacterial and fungal, that have developed spontaneously in adults (1). Other etiologies in adults include arthropathies, such as gout or ankylosing spondylitis, and osseous changes from chronic renal failure or neuropathy (2–6). We present the case of a previously healthy man who presented with radiologic findings consistent with diskitis, which spontaneously resolved without any treatment other than nonsteroidal antiinflammatory drugs (NSAIDs).
Case Report
A 39-year-old man with an unremarkable medical history presented with significant left posterior thorax pain that developed abruptly after a sneeze 2 months earlier. The pain was a constant aching with intermittent periods of severe pain and sometimes fleeting severe pains with certain movements of the torso. There was no history of fever, chills, or night sweats. Social history included recent travel to Japan before the onset of symptoms. Physical examination was unremarkable except for tenderness to palpation at the level of T7. Conventional radiographic images of the chest and ribs were normal. His symptoms were attributed to muscle strain or minor trauma, and the patient was treated conservatively, but the pain persisted. A bone scan was ordered, which demonstrated moderate intensity, abnormal uptake at the posterior left aspect of the T9 vertebral body. As a result of these findings, MR imaging was performed, which demonstrated destruction of the superior endplate of T9 and inferior endplate of T8, with enhancement in the central portion of the disk space consistent with diskitis (Fig 1A and B). Follow-up noncontrast CT demonstrated left pedicle sclerosis, endplate destruction, and slightly irregular lucency at the left lateral cortex of the T9 vertebral body (Fig 1C and D). As a result of these findings, the patient underwent biopsy of the affected disk space. During this time, the patient was treated with a variety of NSAIDs and pain medication, which included Naproxen, Motrin, Ultram (tramadol HCl), and up to five Roxicet (oxycodone HCl/acetomenophin) per day. Follow-up MR imaging 2 months later demonstrated similar findings.
Initial CT and MR images two weeks after the abnormal bone scan as part of work-up for two months of progressive thoracic pain.
A, Initial coronal T1-weighted postgadolinium image with fat saturation demonstrates contiguous intense enhancement of the intervertebral disk, endplate, and adjacent marrow (open arrow), with less intense enhancement of the T8 and T9 vertebral bodies and paraspinous region (arrows).
B, Initial sagittal Fast Spin-Echo T2-weighted and fat-nulled Inversion Recovery (FSEIR) image demonstrates increased signal intensity in the T8 and T9 vertebral bodies and the intervertebral disk (arrow).
C and D, CT coronal (C) and sagittal (D) reconstructions reveal destruction of endplates and adjacent cancelous bone (arrow).
E, Core biopsy needle (arrow) traverses central endplate erosion (arrowhead).
The patient’s laboratory workup demonstrated the following: WBC, 7.4 × 109/L; HGB, 14.3 g/dL; HCT, 41.4%; granulocytes, 80%; lymphocytes, 15%; ESR, 19 mm/h; PSA, 0.33 ng/mL; C-reactive protein, 2.737 mg/dL; blood cultures, negative ×2, HIV screening, negative; RPR, negative; cat scratch, negative; serum coccidoidmycosis screening, negative; and serum histoplasma screening, negative. Biopsies of the disk space were attempted three times. The first yielded precise CT-guided core biopsies of the central disk. The pathologic results confirmed disk, bone, and cartilage fragments with acute inflammatory cells, macrophages, amorphous debris, and rare giant cells (osteoclasts). The second biopsy attempt, 7 weeks after cultures were negative, was unsuccessful because of patient discomfort. The third attempt, 2 weeks later under general anesthesia, produced two core biopsies with similar results to the first biopsy, revealing fibrovascular tissue with chronic inflammation and dense fibroconnective tissue along with fragments of disk, bone, and cartilage (Fig 1E). Gram stain revealed 1+ RBCs and no organisms, and acid-fast stain was negative. Fungal and AFB cultures were negative. Follow-up laboratory results 5 months after presentation revealed WBC, 4.6 × 109/L; HGB, 16.8 g/dL; HCT, 50.1%; granulocytes, 50.7%; lymphocytes, 38.4%; C-reactive protein, 0.058 mg/dL (normal < .5); and ESR, 1 mm/h.
The patient clinically improved with NSAIDs and pain management. Approximately 3 months after presentation, the patient was pain free and remained so at clinical follow-up 10 months after presentation. Follow-up MR imaging performed both 4 and 8 months after initial presentation demonstrated resolution of the inflammatory process. The cause of this inflammatory process could not be determined, and the patient was given the diagnosis of a nonspecific inflammatory process of the vertebral bodies and intervertebral disk.
Discussion
In adults, diskitis most frequently occurs following spinal surgery. These cases are most typically caused by S aureus and S epidermidis. In patients who have spontaneous infectious diskitis, the etiologies are a wide variety of Gram-negative, Gram-positive, and fungal organisms. These patients typically have slow or insidious onset of pain, which may be difficult to differentiate from other causes of back pain. The sensitivity of imaging-guided needle biopsy in the setting of infectious diskitis has been reported to be 58–91%, but the sensitivity decreases in the setting of fungal infections (7).
Also, there are many noninfectious etiologies that can mimic infectious diskitis in adults. These include inflammatory conditions such as ankylosing spondylitis, metabolic conditions, which include those related to renal failure, and neuropathic arthropathy (2–6). Chondrocalcinosis has been known to result in destructive lesions of the vertebral bodies that can mimic infectious or ankylosing spondylitis. The diagnosis of chondrocalcinosis is usually made by demonstration of the crystals, but they can be absent in inflammatory lesions (8).
The typical MR imaging appearance of diskitis includes loss of distinction between the endplates and intervertebral disks on T1-weighted images, with decreased signal intensity in the adjacent vertebral bodies on the T1-weighted images and increased signal intensity on T2-weighted images. The involved disk typically is narrowed and ill defined on T1-weighted images and has increased or mixed signal intensity on T2 weight images (9, 10). In the adult, this process is typically more aggressive than in the child, with frank destruction of the endplates (11).
The processes described above, which are known to cause noninfectious diskitis, are not known to resolve or improve with treatment of the underlying cause, and were not present in this patient. The history of acute onset of pain while sneezing suggests the possibility of an acute intraosseous disk herniation. Seymour et al (12) reported six such cases in 1998. Only two of these cases were associated with a precipitating traumatic event. These cases were evaluated only with noncontrast MR imaging, and all were characterized by persistent endplate defect due specifically to disk herniation. Our case lacks endplate herniation, but rather demonstrates contiguous disk, endplate, and marrow enhancement (Fig 1A). Our case demonstrated eventual endplate destruction more characteristic of diskitis (Fig 1C–E). Finally, our case is distinct in that the endplate spontaneously resolved (Fig 2A–C), whereas both endplate herniations and diskitis typically produce a persistent focal defect or irregularity of the endplate.
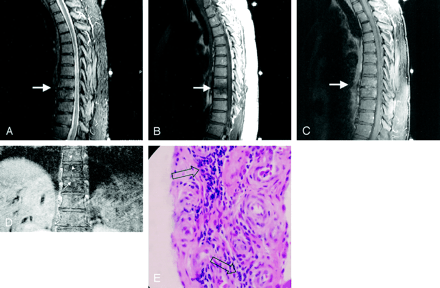
Follow-up MR images obtained 5 months later.
A, Sagittal FSEIR findings are normal, with restoration of intact T8–T9 endplates (arrow) and normal T8 and T9 marrow signal intensity.
B, T1 precontrast image (arrow) shows residual decrease in marrow signal intensity.
C, Postgadolinium fat-saturated sagittal image demonstrates complete resolution of enhancement of the T8 and T9 vertebral bodies and paraspinous tissues and minimal residual enhancement of the T8–T9 intervertebral disk.
D, At 9 months, a Schmorl’s node is evident along the superior T9 endplate (arrow), larger than those present at the level above (arrowheads), which were present on the initial image. (Note in Fig 2D the paraspinous soft tissue swelling and enhancement is gone, compared with Fig 1A.)
E, Biopsy at 4 months reveals chronic mononuclear inflammation surrounding fibrovascular tissue (arrows).
Because of the peculiarly intense enhancement pattern, negative biopsy results, and resolution, one can only speculate as to the actual cause. Perhaps this represents a form of reflex sympathetic dystrophy (RSD), which is known to produce increased blood flow, regional enhancement and juxta-articular erosions (13, 14). Furthermore, it is known that, as RSD symptoms resolve, bone density can normalize (15). It is possible, therefore, that some imperceptible injury to the annulus or disk might have prompted such a reaction and accounts for the multiple abnormalities manifest in our patient.
RSD is primarily a clinical diagnosis, represented by progressive pain disproportionate to an often minor preceding injury, which has been attributed to malfunction of the autonomic nervous system as it often improves after sympathetic denervation (13). It has been described as occurring in three phases, with most patients normalizing after the second phase. Our patient had symptoms characteristic of RSD: severe progressive burning pain with a radicular (intercostal) component. This is typical of symptoms described in phase I RSD, or “warm” phase (13). The time course and symptom resolution (4 months) are also consistent with phase I RSD. MR imaging revealing soft tissue (paraspinous) swelling, edema and enhancement during clinical phase I, which resolve along with the pain in clinical phase II are again consistent with RSD (13).
Biopsies of RSD after the 7th week have revealed osteoclastosis, and biopsies at 3–4 months, reveal chronic inflammatory reaction, edema and hypervascularity of the soft tissue (16–20). Although these biopsy findings in RSD are nonspecific, they are known. These were the findings in the first and second biopsies in our patient, at 2 and 4 months, respectively. To our knowledge, the literature has only one instance of RSD as the proposed cause of progressive pain involving the axial skeleton, in which the diagnosis was proposed based strictly on clinical symptoms (not imaging) following whiplash injury (21). There are three clinical phases of RSD (13, 16, 22). Phase I RSD can last several months and is further characterized by skin thickening, warmth, hyperemia, and edema sometimes removed from the site of initial trauma. Phase II, the “cold” phase, presents later, up to 2 years from onset, and reveals a cold extremity, with vasoconstriction and absence of enhancement on MR imaging (13, 16, 22). Most patients improve, but some go on to phase III, or chronic, RSD, which presents later still with muscle atrophy, glossy skin, spasm dystonia, and tremor.
In light of our patient’s normal-appearing annulus on initial MR images, one might propose that the onset of his pain during a sneeze might have lead to an endplate injury. Diskogenic pain is known to worsen with sneezing (23–25). It is known that sneezing and axial loading elevate the intradiskal pressures (26). Endplate injuries, and resultant endplate intraosseous disk herniations, or Schmorl’s nodes, can frequently occur in normal bone (27). The enhancement of the intact disk and endplate (Fig 1A) on the 2-month and 4-month MR images may relate to a stress fracture of the endplate, which had not yet allowed Schmorl’s node formation. Later, on the final MR images obtained at 9 months (Fig 2D), a residual endplate defect containing normal disk signal intensity (a Schmorl’s node) was evident 5 months after CT (Fig 1C, -D) already demonstrated extensive endplate resorption. It is thus difficult to say whether this Schmorl’s node was the residuum of an initial endplate stress fracture or simple disk occupying the potential space produced by the extensive resorption of bone during the fourth month of symptoms.
The patient’s pain rapidly resolved at 4.5 months from onset of pain. MR imaging during this 4-month period of symptoms remained markedly abnormal (Fig 1A), whereas MR imaging at 6 and 9 months revealed resolution of both marrow edema and paraspinous soft tissue swelling and enhancement. Although extensive marrow edema is known to accompany acute Schmorl’s nodes, it seems our patient’s pain syndrome and imaging findings go beyond these limited manifestations of painful acute Schmorl’s nodes described elsewhere (12, 28). Schmorl’s nodes themselves can enhance, but enhancement in this case crossed the disk space into intact endplates (Fig 1B) and, unlike previous descriptions of acute Schmorl’s nodes, caused enhancement within the entire adjacent vertebral bodies as well as in the secondary finding of paraspinous soft-tissue thickening (28).
Conclusion
The occurrence of severe, debilitating thoracic pain in a healthy middle-aged man warrants careful evaluation. An apparent noninfectious and idiopathic inflammatory condition detected here, which then resolved with only symptomatic treatment, bears a strong resemblance to the syndrome of RSD. Although RSD has been studied with great frequency in the extremities, it is still poorly understood (21, 22). Nonetheless, such an aggressive-appearing process could represent this entity in the spine, provided all other treatable infectious and inflammatory conditions have been excluded.
References
- Received January 31, 2004.
- Accepted after revision August 10, 2004.
- Copyright © American Society of Neuroradiology