Abstract
BACKGROUND AND PURPOSE: The endovascular occlusion of aneurysms with unfavorable configurations such as a broad neck and an important branch from the fundus remains a technical challenge. The purpose of this study was to evaluate the radiologic and clinical results of complicated aneurysm treatment by using two microcatheters.
METHODS: Twenty-five aneurysms in 25 patients were treated by using two microcatheters, from August 2001 to February 2004. Fourteen patients presented with a subarachnoid hemorrhage (SAH) and 11 had unruptured aneurysms. The aneurysms were of the basilar top (7), middle cerebral artery bifurcation (4), posterior communicating artery (4), anterior communicating artery (3), superior cerebellar artery (2), ophthalmic artery (2), and one aneurysm of each of cavernous internal carotid artery (ICA), dorsal ICA, and midbasilar artery. In 16 aneurysms (64%), the width of the aneurysm was the same or longer than the height. In 19 (76%), important branches arose from the aneurysm base, and some were even incorporated with the aneurysm fundus. The mean dome (height)-to-neck ratio was 1.23 ± 0.37 (range, 0.65–2.33), and this was greater than or equal to 1.0 in 19 aneurysms (76%).
RESULTS: All aneurysms were successfully embolized. Immediate postembolization angiography showed no residual contrast filling in eight aneurysms (32%), and some residual contrast filling in 16. The aneurysm remnants, however, were intentionally left to preserve important branches in 12 of the 16 aneurysms with incomplete occlusion. Two complications occurred, including one thromboembolic and one coil protrusion, but they were successfully resolved and produced no clinical symptoms. All patients except one showed excellent clinical outcomes. One patient revealed moderate cognitive dysfunction. During the follow-up period, no new bleeding occurred.
CONCLUSION: Our experience with 25 cerebral aneurysm patients shows that the technique of using two microcatheters is feasible and safe for coil embolization of aneurysms with unfavorable configurations. Although the lack of angiographic follow-up prevents us from drawing conclusions about its effectiveness as compared with other techniques such as stent placement and balloon-neck protection, we believe that this technique offers a reliable alternative for endovascular therapy of complicated aneurysms.
Despite advances in devices and techniques, there remain some cerebral aneurysms difficult or impossible to treat with coils. Unfavorable aneurysm configurations, such as a wide aneurismal neck and major branches incorporating with the aneurysm fundus, are the main technical limitations (1). Advanced techniques using a balloon or a stent can be applied, but the introduction of additional devices into small intracranial vessels is not only technically demanding, but also may increase the risk of vascular injury (2–7). Moreover, these techniques may be unsuitable for some aneurysms, especially those with important branches arising from the fundus.
For several years, we have used two microcatheters instead of a stent or a balloon for coil embolization of cerebral aneurysms with complicated configurations. The key concept of this technique is making a stable coil frame with two coils through two microcatheters. The use of two microcatheters for aneurysm embolization is not a new concept. In 1998, Baxter et al (8) reported two such cases. In this report, we present our experiences of using two microcatheters in 25 patients and describe its techniques, efficacy, safety, and possible disadvantages.
Methods
From August 2001 to February 2004, a total of 25 aneurysms in 25 patients were treated by using the two-microcatheter technique at Seoul National University Hospital and at Seoul National University Bundang Hospital. During this period, 415 cerebral aneurysm patients were treated by coil embolization. Twenty aneurysms were treated with the stent-assisted technique, and four were treated with the balloon-protection technique. Two microcatheters were used for more than 40 cases of aneurysms; however, 25 cases in which both microcatheters were used for coil deployment are included in this report. In most of the excluded cases, one microcatheter was used not to deliver coils, but rather to protect an important branch from the aneurysm neck.
The angiography units, biplane Integris Allura systems (Phillips Medical Systems, Best, the Netherlands), were used. The size of each aneurysm was measured in 3D angiographic images by using the angiography unit software. The width of an aneurysm was determined by measuring the longest diameter of the fundus parallel to the axis of the aneurysm neck. The height was determined as the longest diameter of the fundus vertical to the axis of the aneurysm neck. Therefore, if the width-to-height ratio exceeded 1, the aneurysm was lying transversely on the parent artery.
Of the 25 patients, 13 were female and 12 were male. The mean age of the patients was 55 ± 11.6 years (range, 33–80 years). Fourteen patients presented with SAH and 11 had unruptured aneurysms. Patient age and sex, the location and size of the aneurysms, the mode of presentation, and clinical outcomes are summarized in Table 1. There were seven basilar top aneurysms, four middle cerebral artery bifurcation aneurysms, four posterior communicating artery aneurysms, three anterior communicating artery aneurysms, two superior cerebellar artery aneurysms, two ophthalmic artery aneurysms, and one of each of cavernous internal carotid artery (ICA), dorsal ICA, and midbasilar artery aneurysm. The mean size of the aneurysm neck was 8.11 ± 3.42 mm (range, 3.3–16.0 mm). Only one aneurysm had a neck of less than 4 mm. The mean width of the aneurysm dome was 10.5 ± 4.5 mm (range, 4.3–24 mm), its mean height was 10.0 ± 5.0 mm (range, 2.6–19 mm), and the mean width-to-height ratio was 1.2 ± 0.5 (range, 0.5–2.3). In 16 aneurysms (64%), the width of the aneurysm was the same or longer than the height. In 19 aneurysms (76%), important branches arose from the aneurismal base or were even incorporated with the aneurysm fundus. The mean dome (height)-to-neck ratio was 1.23 ± 0.37 (range, 0.65–2.33). The dome-to-neck ratio was greater than or equal to 1 in 19 aneurysms (76%).
Summary of the 25 patients with 25 aneurysms
In all patients coil embolization was performed as the primary treatment. A technique using two microcatheters was chosen after the failure of a conventional single-microcatheter technique in seven cases. In the remaining 18 cases, the two-microcatheter technique was adopted from the beginning. Immediate postembolization angiography was performed in all patients. The angiographic results were interpreted and classified somewhat arbitrarily as complete occlusion (no residual contrast filling) or incomplete occlusion (any residual filling). Incomplete occlusion was further classified as neck remnant, fundus remnant, or intentional remnant. “Intentional remnant” was defined as postembolization angiography showing residual contrast filling in a part of the aneurysm, but when this part was intentionally not occluded to preserve an important arterial branch, which was incorporated with the aneurysm.
Outcomes were assessed at the last clinic visit by using the modified Rankin scale: 0, no symptoms at all; 1, no significant disability despite symptoms and able to carry out all usual duties and activities; 2, slight disability and unable to carry out all previous activities, but able to look after own affairs without assistance; 3, moderate disability requiring some help, but able to walk without assistance; 4, moderately severe disability, unable to walk without assistance and unable to attend to own bodily needs without assistance; 5, severe disability, bedridden, incontinent, and requiring constant nursing care and attention; and 6, dead.
Techniques
Following the induction of general anesthesia, a 6F sheath was placed in the common femoral artery. In most cases, one guiding catheter (6F) was used. Bilateral femoral routes with two guiding catheters (5F or 6F) were used for our earlier cases, but here two arterial punctures are made only in cases of basilar artery aneurysms with small vertebral arteries that cannot accept a 6F guiding catheter.
A 3000-U heparin bolus was administered after femoral arterial sheath placement in the case of an unruptured aneurysm and after deployment of the first or second coil in the case of a ruptured aneurysm. Intermittent boluses of 1000 U/h heparin were then administered.
Preparation and assembly of devices is basically similar to that of conventional coil embolization procedure. To use two microcatheters through a single guiding catheter, we attached a rotating hemostatic valve that has three-side ports (one for flushing a guiding catheter and two for microcatheters) or two conventional rotating hemostatic valves connected in line fashion.
With diagnostic angiography and 3D angiographic reconstruction, the morphologic characteristics of the aneurysm were carefully inspected, such as the size of the neck, width, and height of the aneurysm fundus; the presence of any bulging or depressed parts, which can be helpful in coil stability; and the anatomic relationship with the parent artery and major branches to be preserved. Detailed strategies of the coil embolization technique mainly depended on angiographic findings. 3D angiographic images were used to measure the various dimensions of each component. On the basis of the above characteristics, detailed planning was undertaken including the optimal projection, microcatheter positioning, selection of the coil size and shape, and sequence of the coil detachment. The aneurysm was then selected by using the two microcatheters one by one; 14 or 10 series of microcatheters were used. In general, commercially available 6F thin-walled guiding catheters have enough inner space to inject contrast media for angiography even with two 14 series microcatheters inside the lumen.
In practice, it is important to distinguish between the two microcatheters in fluoroscopic views during the procedures. Therefore, we used a combination of microcatheters with different-shaped distal markers. Prowler-10 (Cordis, Miami, FL) and Excelsior SL-10 (Boston Scientific, Fremont, CA) were a common combination in our series.
Technically, the basis of the coil stability should be achieved with the first two coils. Accordingly, at the initial stage of the procedure, detailed technical considerations were focused on coil selection (size, length, softness, and shape of the first two coils) and microcatheter positioning.
In general, the aneurysm sac was divided into two imaginary parts, and each part was occupied by the first and second coils. These imaginary parts need not be half of the aneurysm. Coil size (diameter) was selected by the sizes of these imaginary parts. In transversely lying aneurysms, the maximum coil size was selected according to the short length (height) of the aneurysm. The type of coil configurations—such as 2D or 3D—was selected to occupy each part and to intermingle with each other in the area adjoining the two parts. Regarding coil length, to the extent aneurysm volume allowed, the longest coil was selected to make more stable coil frame and reduce the number of subsequent coils required for compact packing. Soft coils were commonly used for especially dangerous-looking aneurysms, such as acutely ruptured ones, small-sized ones, and aneurysms with complicated configurations for coils to move smoothly (such as multiple deep indentations and daughter sacs). The coils used in our series included various types of Guglielmi detachable coils (GDCs; Boston Scientific; standard helical, 2D, 3D, standard, soft, ultrasoft), Microplex coils (MicroVention, Aliso Viejo, CA), J-shaped Detach-DCS (Cook Europe, Bjaerskov, Denmark), and Trufill-DCS Complex (Cordis).
In general, the second coil was deployed after complete deployment, but before detachment, of the first coil. Sometimes, to make a more complicated mixture of the two coils, several centimeters of each coil were deployed alternatively. After complete deployment of the two coils, more stable-looking coil was detached first. Sometimes, however, the more stable-looking coil was not detached first, especially when it formed a stable large frame that prevented excessive movement of the second small coil, which remained within this frame.
Because only one coil is detached and the other coil is left undetached, one additional coil (the third coil) is used for adding more stability. After making a stable coil frame by using two or three coils, the residual aneurismal sac was filled with small coils through both microcatheters. After completing coil packing, the microcatheters were withdrawn and postembolization angiography was performed. Figures 1–3 show technical details of the two-microcatheter technique.
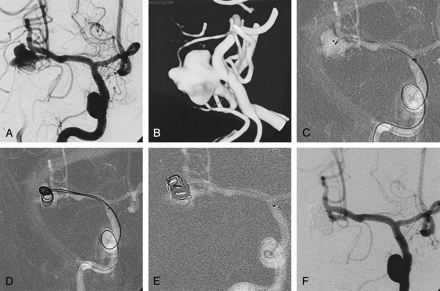
Case 1, a 54-year-old man with SAH.
A, Right internal carotid angiogram shows a large aneurysm of middle cerebral artery bifurcation. The inferior division (M2) is incorporated with the neck.
B, The aneurismal configurations are visualized in 3D image.
C, Two microcatheters with different markers are positioned within the aneurysm. The microcatheters have different distal shaping.
D, Two coils (GDC-10; 5 mm × 15 cm) deployed via two microcatheters. Each coil occupies different parts of the aneurysm, and simultaneously they are mixed at the central part. In this case, to make a more complicated mixture of the two coils, the second coil is being advanced before complete deployment of the first coil.
E, After detachment of the first coil, the third coil advanced within the aneurysm. The second coil is not detached until a more stable coil mass is obtained.
F, Angiogram obtained immediately after embolization shows compact occlusion of the aneurismal sac and patent inferior division (M2). There is small neck remnant around the inferior division.
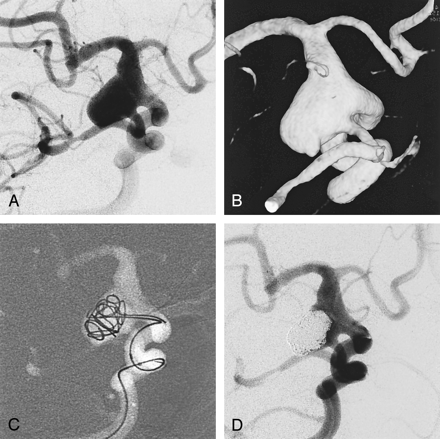
Case 12, an 80-year-old woman with Hunt and Hess grade 4 SAH.
A, Angiogram shows a large aneurysm of fetal type posterior communicating artery.
B, 3D image of the same aneurysm.
C, Two coils (GDC-10–2D; 7 mm × 25 cm, 5 mm × 15 cm) are being deployed via two microcatheters.
D, Final angiogram shows residual neck to preserve the fetal type posterior communicating artery. The dome of the aneurysm is compactly occluded.
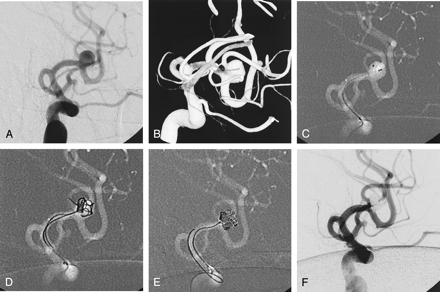
Case 25, a 38-year-old man with Hunt and Hess grade 2 SAH. Initially, surgical clipping had been planned, but embolic occlusion of MCA branches occurred during diagnostic angiography. Right upper-extremity monoplegia and motor dysphasia developed. For rescue thrombolysis, urgent endovascular occlusion of the aneurysm was performed.
A, Angiogram shows an aneurysm of MCA bifurcation. The superior division (M2) arises from the fundus of the aneurysm.
B, Clearer aneurismal configurations are viewed in 3D image.
C, Two microcatheters are placed within the aneurysm. Note the position of the microcatheters.
D, To protect the superior division (M2), several centimeters of a small coil (GDC-10-soft-SR; 2 mm × 8 cm) are deployed first through one microcatheter near the origin of the superior division (arrow), and this coil is interfering with movement of the second coil (GDC-10–3D; 3 mm × 8 cm) toward the superior division (M2).
E, After achieving a stable coil frame without compromise of the M2, the first coil that had been used for protection of the superior division was carefully retrieved without movement of the coil frame, and, after changing the microcatheter position, it was reinserted into the coil mass.
F, Final angiogram shows complete occlusion of the aneurysm and patent both M2. After the procedure, the patient recovered without neurologic deficit.
In a case with an middle cerebral artery (MCA) bifurcation aneurysm (case 25, Fig 3), a somewhat different embolization technique was applied. In this case, the superior division of M2 arose from the fundus of the aneurysm. To protect this critical artery, a small coil was deployed first through one microcatheter near the origin of the superior division, and this coil interfered with movement of the second coil toward the superior division (M2). After a stable coil frame had been formed without compromise of the M2 by the second and subsequent coils, the first coil was retrieved and reinserted into the stable coil mass for sac filling (Fig 3).
Results
All aneurysms were successfully embolized. Immediate postembolization angiography showed no residual contrast filling in eight patients (32%) and some residual contrast filling in 16. In 12 of the 16 patients with incomplete occlusion, however, remnant parts were left intentionally to preserve important branches.
There were two technical complications, including one thromboembolic and one coil protrusion. In the thromboembolic complication case (case 2), thrombi were found around the coil mass at the end of the procedure, but they were completely resolved by the intraarterial infusion of abxicimab. In one case of large anterior communicating artery aneurysm (case 16), a short segment of the previously detached coil protruded into the parent artery (A1) by coil jamming during the repositioning of the subsequent coil, but the protruded coil segment was short and did not interfere with blood flow or cause any thromboembolic problem. Neither aneurismal rupture nor occlusion compromise of the parent or branching arteries occurred.
The duration of the clinical follow-up ranged from 1 to 26 months after the initial procedure, with a median of 7.9 ± 6.9 months. No new bleeding occurred during the follow-up period. All except one patient returned to their previous work and remained symptom-free (modified Rankin scale 0). One male patient (case 13) presented with Hunt and Hess grade 4 SAH had moderate cognitive dysfunction and could not return his previous work (modified Rankin scale 3).
Follow-up angiography was performed in six patients (case 9, after 12 and 24 months; cases 14, 17, and 19, after 12 months; case 21, after 8, 12, and 24 months; and case 23, after 3 months). Coil compaction and partial recanalization of the neck were found in three patients (cases 6, 9, and 21). Repeated embolization was performed in two patients (cases 9 and 21) by using a conventional single-catheter technique without complication. In case 6, retreatment was not attempted because the recanalized space was minimal.
Discussion
The technique of using two microcatheters has several advantages over the stent or balloon-assisted techniques. First, the devices required are the same as those for the conventional coil embolization. No additional femoral puncture is required, and one 6F guiding catheter is used as is conventional. The only difference is the use of two microcatheters, but the introduction of an additional microcatheter is not technically demanding. The risks directly associated with devices are low. In addition, by not using relatively bulky instruments, this technique can be applied even for small distal tortuous vasculatures, provided that the vessel can contain the two microcatheters. The outer diameter of the distal segment of commercially available 10-series microcatheters is usually 1.7F (0.57 mm). One may assume that placing two 10-series microcatheters is the same as placing one 3.4F (1.13 mm) catheter, but, because the catheter is round, the cross-sectional area occupied by the two microcatheters is about one-fourth (0.25 mm2) of that occupied by one 3.4F catheter (1.01 mm2). The same principle can be applied to the guiding catheter. Even with two microcatheters within a guiding catheter, the residual guiding catheter lumen provides sufficient space for road mapping or angiography. By calculation, the cross-sectional area of the residual luminal space of conventional 6F guiding catheter that is occupied by two microcatheters of 10- or 14-series is similar to that of a conventional 5F diagnostic catheter.
The most important technical point of this technique is the achievement of coil stability with the initial two coils without parent artery or major branch compromise. Various factors can influence coil stability, including the position of the microcatheters, coil size, 3D coil characteristics, morphologic features of the aneurysm affecting coil stability, and so on. Theoretically, the combination of these various factors may be almost infinite. Of these numerous factors, the catheter factors and the coil factors are controllable, and we have learned that the coil factors are more critical than catheter factors. Placing the microcatheters at the exact point at each imaginarily divided part of the aneurysm is often difficult or impossible. Unsatisfactory catheter positioning can often be overcome by using coils of the appropriate size, length, and shape, Therefore, in technical terms, the appropriate selection of the first two coils is important. In addition, because only one of these coils will be detached, the subsequent 3rd coil can also be used to add stability.
Coil deployment technique is also important. In general, the second coil is deployed after complete deployment of the first. This is recommended because several attempts of coil advancement and retrieval are often required to achieve a stable coil frame, and this procedure may have a potential risk of coil sticking and stretching. The interlocking of the two coils may cause serious difficulties in terms of coil advancement or retrieval. It may be fortunate that we have not encountered coil interlocking or stretching in real practice. But we have not been greatly concerned about these problems, because the initial two coils can be controlled or even completely retrieved as long as they are not detached.
After achieving coil stability with the initial two coils, one coil is detached while the other one is left undetached. Determining which coil should be detached first may be a difficult decision. In general, the more-stable-looking coil is detached first. The sequence of coil detachment may, however, vary with situations. Sometimes the less-stable-looking coil is detached first, especially when a large coil forms a stable large frame that prevents excessive movement of the second small coil, which remains within the stable coil frame. If there are critical concerns regarding the coil mass stability even after the initial two coil deployment, an additional arterial sheath is inserted at the opposite femoral artery, a second guiding catheter is placed, and a third microcatheter is placed within the aneurysm. An additional coil may be inserted to stabilize the coil mass, although, in our experience, a third microcatheter has not been required. After making a stable coil frame with two or three coils, the residual aneurismal sac was filled with small coils through both microcatheters.
During our earlier experiences with this technique, one of our major concerns was whether complicated-looking aneurysms could be embolized successfully and safely by this method. It is noteworthy, however, that all of the aneurysms treated produced satisfactory outcomes. We have not yet experienced a case of technical failure, although we do not believe that this technique can be applied to all complicated aneurysms. In our experience, regardless of neck size, large aneurysms—because they provide sufficient space to manipulate coils—and aneurysms that have localized bulges or depressions on which coils can stably stand are more suitable for this technique.
We do not believe that this has higher risk of procedure-related complications than the conventional single-microcatheter technique. As our series shows, technical complications rarely occurred. In fact, the two technical complications in our series are not believed to be directly related to the technique by using two microcatheters. The relatively high cost of using a greater number of coils may be a potential disadvantage of this technique. In this technique, the initial coil frame is made within the aneurysm by using two coils—that is, the aneurysm is divided into at least two parts from the beginning. Each part is packed with coils smaller than the size of the entire aneurysm. Therefore, more coils tend to be used as compared with the conventional single-catheter technique, in which coil size is selected according to the long diameter of the aneurysm.
Conclusion
Our experience with 25 patients with cerebral aneurysms shows that the two-microcatheter technique is a feasible and safe technique for the coil embolization of aneurysms with complicated configurations. Its technical details are more than an extension of conventional embolization, but they are relatively simple. No specific additional device requiring special education is used. It simply involves introduction of one more microcatheter into the aneurysm.
Although more data on long-term results will be required, we believe this technique may offer a reliable alternative for the endovascular therapy of aneurysms with complicated configurations.
References
- Received May 26, 2004.
- Accepted after revision August 24, 2004.
- American Society of Neuroradiology