Abstract
BACKGROUND AND PURPOSE: We evaluated a mechanical thrombectomy protocol to treat acute stroke and report the angiographic results and clinical outcomes.
METHODS: Patients with anterior circulation strokes <8 hours and posterior circulation strokes <12 hours were treated at a single center over 10 months. Patients were excluded if they were candidates for intravenous tissue plasminogen activator (tPA). Treatment involved one of two mechanical thrombectomy devices. Retrieval was augmented by low-dose intra-arterial tPA if needed. Outcome was measured by using the Modified Rankin score.
RESULTS: Ten patients were treated: five with anterior circulation strokes, four with posterior circulation strokes, and one with embolic strokes involving both circulations. Mean National Institutes of Health Stroke Scale score at presentation was 24.6 ± 10.9. In eight patients (80%), revascularization was successful (Thrombolysis in Acute Myocardial Infarction score, 3). Mean time from symptom onset to initiation of the procedure was 6 hours (5.3 hours for anterior circulation and 7.0 hours for posterior circulation). Mean time for recanalization from the start of the procedure was 1.17 ± 0.58 hours for the six anterior circulation strokes and 2.75 ± 1.34 hours in the two posterior circulation strokes. Five patients died within 48 hours; all had posterior circulation strokes. Mean Modified Rankin score at 90 days was 1.4.
CONCLUSION: In this small series, mechanical thrombectomy of acute stroke appeared to improve recanalization rates compared with intra-arterial thrombolysis. No hemorrhagic complications occurred. Further study is required to determine the role of these techniques.
Endovascular treatment of acute stroke by using intra-arterial (IA) thrombolysis has been reported in a randomized controlled trial (1, 2). The Pro-Urokinase for Acute Cerebral Thromboembolism (PROACT) II trial showed that a significantly more patients with middle cerebral artery (MCA) strokes were able to live independently at 3 months when treated with IA thrombolysis compared with placebo (1). Despite this benefit the risk of hemorrhage with thrombolytic therapy is 10% versus 2% with controls, and the time to recanalization is often 2 hours. In addition, although the rate of recanalization is 66% for IA thrombolysis (Thrombolysis in Acute Myocardial Infarction [TIMI] grade, 2 and 3), the rate of complete recanalization is 19% (TIMI grade, 3) (1, 2).
Mechanical thrombectomy is being considered to improve the rate and speed of recanalization and possibly decrease the incidence of symptomatic hemorrhage. Mechanical thrombectomy has been described in case reports and a small case series (3–6). A protocol was recently adopted at our institution in an attempt to use primarily mechanical thrombectomy in the endovascular treatment of acute thromboembolic strokes rather than IA thrombolysis. The purpose of this study was to describe the results for treatment in the first 10 patients treated with this protocol.
Methods
We retrospectively reviewed 10 patients who were treated after the adoption of a mechanical thrombectomy protocol for acute ischemic stroke between November 2002 and August 2003 (10 months). All patients at our institution referred for endovascular therapy after adoption of this protocol were primarily treated with mechanical thrombectomy. Thrombolytic therapy could be used if the primary therapy was thought to provide inadequate recanalization. Patients were considered for mechanical thrombectomy for up to 8 hours after ictus in cases of anterior circulation strokes and for up to 12 hours in cases of posterior circulation strokes. Patients had to have a National Institutes of Health Stroke Scale (NIHSS) score of greater than 8. Patients presenting at 0–3 hours were excluded if they were candidates for intravenous (IV) tissue plasminogen activator (tPA). Time from symptom onset to presentation was recorded in all patients.
A stroke neurologist who performed the initial and subsequent neurologic examinations referred the patients. Screening CT was performed in all patients to ensure that no evidence of hemorrhage or large region of hypoattenuation (>1/3 of the MCA territory) was present before the start of endovascular therapy.
Patients were transported to the angiography suite, where an angiogram was obtained by using conscious sedation with a 6F sheath and a 5F diagnostic catheter. The arterial territory initially selected was the one suspected of being thrombosed. Endovascular treatment was carried out with systemic heparinization (5000-U bolus followed by 1000 U/hour). Postprocedural anticoagulation was not used; however, heparin therapy was not immediately reversed with protamine sulfate.
Patients were treated with one of two devices: the Concentric Retriever thrombectomy device (Concentric Medical, Mountain View, CA) or the Neuronet Retriever thrombectomy device (Guidant Corporation, Indianapolis, IN). The US Food and Drug Administration (FDA) approved the Neuronet device for use in foreign body retrieval. The device is a flexible, tapered core wire with a flexible retrieval basket attached to its distal tip. We obtained approval from our institutional review board to use the Concentric Retriever. This device is also a flexible, tapered core wire with helical loops at its distal end that assume a corkscrew-like appearance. Patients treated with the Concentric device were enrolled in a nonrandomized mechanical thrombectomy trial sponsored by the FDA (6 cases). The Neuronet device was used in four cases: in three cases in which the patient could not be enrolled in the FDA trial and in one case in which a physician decided to use it on the basis of a previous positive experience. Retrieval was augmented by low-dose IA tPA (<10-mg total dose) if revascularization was considered suboptimal with the thrombectomy device alone.
The goal of thrombectomy was to achieve a complete recanalization (TIMI grade, 3). The degree of occlusion before and after thrombectomy was measured by using the TIMI scale. The interventional neuroradiologist who performed the procedure recorded the score. Recanalization time was the time to attain flow of TIMI grade 3. Outcome was measured by using the NIHSS and Modified Rankin scores. The NIHSS measures neurologic deficit in 11 neurologic categories, with measures that range from 0 to 42, with 0 representing no neurologic deficit (7). The NIHSS score was determined at presentation and at 24 hours, 5 days, 30 days, and 3 months after the procedure. The Modified Rankin score was measured at 5, 30, and 90 days. All patients underwent CT at 24 hours to assess for hemorrhage.
Results
During the study period, 11 patients with acute stroke were examined at our institution within an appropriate time for treatment. One patient was enrolled in a National Institutes of Health (NIH) trial of IV therapy and therefore not treated. One woman and nine men were treated. Their mean age was 62 years. They had five strokes in the anterior circulation (MCA and/or internal carotid artery [ICA]), four in the posterior circulation (vertebral or basilar), and one stroke in both anterior and posterior territories. Six patients were treated with the Concentric Retriever, and four were treated with the Neuronet retriever. The Neuronet device was successful in establishing flow in three patients, and the Concentric Retriever device was successful in five (Fig 1). Three patients required IA tPA (mean dose, 9.7 mg) to aid revascularization. No symptomatic hemorrhages occurred after treatment.
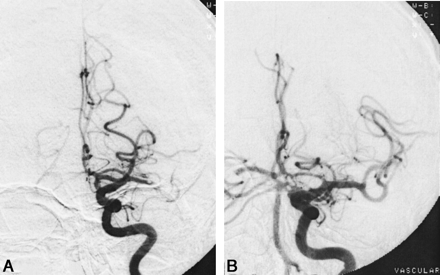
A 77-year-old man with right-sided weakness and aphasia (NIHSS score, 13) underwent angiography 2.9 hours after the onset of his symptoms.
A, Initial angiogram shows a left MCA occlusion.
B, After five passes of the Concentric device and 10 mg of tPA, image shows complete recanalization 4.6 hours after initial onset. At 90-day follow-up, he was symptom-free, with a Modified Rankin score of 0.
The table shows the locations of the lesions and treatment results, with the patients’ initial NIHSS and outcome measures. Eight (80%) of 10 thromboses were successfully recanalized (TIMI grade, 3). The two not revascularized were both posterior circulations strokes, and in both cases, the procedure was terminated. In one case, the Concentric device could not access the lesion because of tortuousity near the lesion. In the other case, microwire perforation occurred after two unsuccessful attempts with the Neuronet device. Final angiograms were also inspected for distal emboli. No cases of angiographically demonstrable distal embolizations occurred.
Patient data
The mean time from symptom onset to the initiation of endovascular therapy was within 6 hours. Six patients were treated in less than 6 hours. The mean time to treatment was 5.3 hours in cases involving the anterior circulation and 7.0 hours in those involving the posterior circulation.
Two of four patients treated after 6 hours from symptom onset had a greater than 7-point improvement in their NIHSS score at 30 days. In one patient with an MCA stroke who was treated at 8 hours, the NIHSS score changed from 9 to 1 at 30 days, with a Modified Rankin score of 0 at 90 days. In another patient, who had an anterior circulation stroke and who was treated at 7.5 hours, the NIHSS score change from 16 to 8 at 30 days; the 90-day Modified Rankin score was 4. Two deaths occurred in the group treated beyond 6 hours, and both patients had strokes involving the posterior circulation. One patient had an initial NIHSS score of 37 and recanalization was not accomplished; the other presented with an NIHSS score of 38.
The mean time to establish TIMI grade 3 flow from the start of the procedure was 1.4 hours ± 0.7 (range, 0.2–2.7 hours). The mean time to establish TIMI grade 3 flow was 1.17 hours ± 0.58 (range, 0.2–1.8 hours) for anterior circulation strokes and 2.75 hours ± 1.34 (range, 1.8–2.7 hours) for posterior circulation strokes. In the eight patients in whom recanalization was successful, mean time from onset of symptoms to restoration of TIMI grade 3 flow was 7.3 hours ± 2.2. In both the cases treated with the Concentric device and those treated with the Neuronet device, the average number of passes to achieve TIMI grade 3 recanalization was three (range, one to five). The mean number of passes did not differ between the two devices. Flow arrest was used when the Concentric device was used (n = 6). Flow arrest was not used with the Neuronet device.
Five deaths occurred, all within 48 hours of the original ictus. All of these patients had posterior circulation strokes. Two deaths occurred in patients in whom recanalization was not achieved. The third death occurred in a 48-year-old man who had embolic strokes in both the anterior and the posterior circulations. The emboli occurred during diagnostic cerebral angiography to evaluate subarachnoid hemorrhage. The patient’s clinical condition deteriorated during angiography, the results of which the diagnostic neuroradiology team performing the study interpreted as aneurysmal rebleed. Repeat CT did not show a rebleed, and review of the angiogram several hours later (when the neurointerventional team was consulted) showed evidence of multiple embolic events. Only one large vessel occlusion (in the MCA) was present at the time of repeat evaluation. The MCA embolus was recanalized within 6.2 hours of symptom onset. However, evidence also showed vertebrobasilar emboli with slow flow in the poster inferior cerebellar artery (PICA) circulation. Despite this finding, no large-vessel occlusion was noted in the posterior circulation at the time of our evaluation. Follow-up imaging showed multiple infarcts in the cerebellum, brainstem, and right MCA territory.
A fourth death occurred in a 66-year-old man who was treated at 9.5 hours; he had presented with an NIHSS score of 38 due to thrombosis of the basilar artery. Recanalization was accomplished within 1.8 hours, with five passes of the Neuronet device and the coincident use of tPA 5 mg IA. However, he died within 24 hours. A fifth patient was a 65-year-old man who was treated at 5.9 hours. He had an NIHSS score of 38 as a result of basilar artery thrombosis. In five passes, the Concentric device successfully revascularized the thrombosis. Additional thrombus in both the posterior cerebral arteries and in the left superior cerebellar artery was cleared with a total of 10 mg of IA tPA.
For the study group, the mean NIHSS score at presentation was 24.6 ± 10.9 (range, 9–38). The mean NIHSS score in the five survivors at presentation was 15.2 ± 4.5 (range, 9–21), and the score in the five deceased patients was 34.0 ± 5.1 (range, 33–38). Three of five surviving patients had a Modified Rankin score of 0 at 90 days.
Discussion
We report a high rate (80%) of complete (TIMI grade 3) recanalization in our patient population. This compares favorably with available data from the PROACT II trial, which demonstrated a 66% rate of TIMI grades 2 and 3 recanalization and a 19% of TIMI grade 3 recanalization. In addition, mechanical recanalization was accomplished in a mean time of 1.2 hours, shorter than the 2 hours thrombolytic time in the PROACT II trial.
IV thrombolysis is an effective therapy when initiated within a narrow 3-hour window (8). However, the symptomatic cerebral hemorrhage rate is relatively high, at 6.4% versus 0.6% for placebo (8, 9). Therapy with IV tPA in the 3–6-hour window was not beneficial in the European Cooperative Acute Stroke Study (ECASS) I, ECASS II, and Alteplase Thrombolysis for Acute Noninterventional Therapy in Ischemic Stroke (ATLANTIS) trials (10–12).
The PROACT II trial showed benefit to intracranial thrombolysis after 3 hours (1). A significantly greater percentage of patients were able to live independently at home at 3 months (40% vs 25%) when treated at up to 6 hours with IA thrombolysis compared to placebo. However, symptomatic cerebral hemorrhage occurred in 10%, versus 2% in the placebo group.
Although our study group was small, no symptomatic cerebral hemorrhages occurred. Conservative use of thrombolytic agent in our patient group may have positively influenced this observation. Only three patients required tPA, with an average dose of 9.7 mg.
Case reports of mechanical thrombectomy have recently appeared in the literature. Removal of thrombus was successful in four reports with a total of six patients. Fourie and Duncan (3), Chopko et al (4), and Schumacher et al (5), reported favorable outcomes in their respective case series, describing the use of a snare device in their single-patient experiences. Mayer et al (6) described success using the Neuronet device in three patients with basilar artery thrombosis when flow arrest was used.
Other forms of nonthrombolytic therapy have been reported in the literature and include rheolytic thrombectomy with the Angiojet (Possis Medical, Minneapolis, MN) device in three patients (13) and thromboaspiration six patients (14, 15). Transluminal angioplasty has a reported technical success of 80% in cases with no hemorrhagic complications (16). However, some believe that angioplasty is best reserved for cases in which thrombolysis alone fails (17). Angioplasty combined with thrombolysis improved outcomes in a small group of survivors who had thrombolysis-resistant lesions, as compared with a control group without angioplasty (17). It has been pointed out that angioplasty may propagate a clot distally (18). One group reported that two-thirds of angioplasty cases treated in the setting of acute stroke have angiographic evidence of distal emboli (19).
Failure of recanalization was associated with death in two of our patients. An additional death occurred in a patient with a vertebrobasilar embolic event that was not amendable to treatment. This patient also had an acute subarachnoid hemorrhage. Poor neurologic condition at presentation resulted in a poor outcome. In addition, patients with an NIHSS score of 30 or greater did poorly, as all four died.
Patients treated in the PROACT II trial and in the National Institute of Neurological Disorders Study (NINDS) study had mortality rates of 25% and 12.5%, respectively. Differences between those series and ours are substantial. For instance, their patients’ initial stroke severity differed from those in our small series. Our patients had a mean initial NIHSS score of 25 versus 17 in PROACT II and 14 in the NINDS study. In addition, patients in PROACT II and NINDS were treated within 6 and 3 hours, respectively, whereas our patients were treated up to 8 hours if they had a stroke in the anterior circulation and 12 hours if they had a stroke in the posterior circulation. The increased mortality rate compared with that observed in the PROACT II trial can be explained by the longer window for treatment, the inclusion of sicker patients, and the inclusion of patients with posterior circulation strokes.
Conclusion
Our early experience suggests that acute stroke therapy with mechanical thrombectomy is more effective and faster than IA thrombolysis for producing recanalization. Our 80% complete recanalization rate and 1.2-hour mean procedure time in the anterior circulation exceeded what was achieved with IA thrombolysis in the PROACT II trial. We did observe a high mortality rate in this series. An initial NIHSS score greater than 30, location of stroke in the posterior circulation, and prolonged time from symptom onset to the initiation of therapy were predictive of death or a poor clinical outcome.
References
- Received April 23, 2004.
- Accepted after revision July 16, 2004.
- American Society of Neuroradiology