Abstract
Summary: Cerebral venous thrombosis (CVT) can be difficult to diagnose because of its wide spectrum of clinical manifestations. Its diagnosis may be further complicated when patients initially present with acute subarachnoid hemorrhage (SAH). We report on four patients with SAH revealing a CVT and discuss the role of imaging for diagnostic and pretherapeutic workup. In three women and one man presenting with severe headaches, images initially suggested SAH with no associated parenchymal bleeding. In all patients, SAH involved the sulci of the convexity and spared the basal cisterns. Digital subtracted angiography showed occlusion of intracranial venous sinuses but did not reveal any other cause of SAH. All patients improved with anticoagulant therapy. Risk factors for CVT and SAH, namely, head trauma and oral contraception, were identified in two patients. These cases highlight the fact SAH may reveal a CVT, which should be considered in the diagnostic workup of SAH, especially when the basal cisterns are not involved.
Subarachnoid hemorrhage (SAH) associated with cerebral venous thrombosis (CVT) is rarely reported in the literature (1–7). Acute SAH suggests the presence of a vascular lesion, such as ruptured aneurysm, and CVT is not generally considered in the diagnostic workup of SAH. Surprisingly, patients with CVT and radiologic signs of SAH are seldom reported, although RBCs are commonly observed in the CSF of patients with CVT (8, 9). In a series of 10 patients with CVT who presented with thunderclap headaches, SAH was confirmed in only one patient after CT and an analysis of CSF (3). To date, six patients with CVT and a CT-based diagnosis of SAH have been reported; in three, CVT was associated with hemorrhagic infarction (1, 3–7). We report on four patients presenting with SAH as the initial manifestation of CVT and discuss the importance of imaging workup in preventing delayed diagnosis and in ruling out other potential causes of SAH.
Case Reports
Case 1
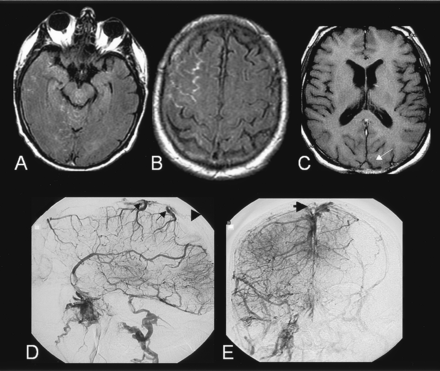
A 69-year-old man with a history of migraine, hypercholesterolemia, and deep venous thrombosis of the left lower extremity at age 50 years presented with sudden onset headaches, which partially regressed with use of anti-inflammatory medication. Results of a lumbar puncture were unremarkable except for 23 RBCs/mm3. Findings on emergency MR imaging, including gradient-echo T2-weighted, fluid-attenuated inversion recovery (FLAIR), diffusion-weighted sequences and 3D time of flight (TOF) MR angiography of the intracranial arteries, were considered normal. Two days after the onset of symptoms, the patient’s neurologic status worsened. As shown in Figure 1, a second study showed hyperintensities on FLAIR MR images and hypointensities on gradient-echo images. These findings were within the subarachnoid spaces of the right frontal convexity, compatible with an acute SAH. The basal cisterns and the parenchyma were normal. MR angiograms of the circle of Willis were still unremarkable, but signal intensity changes in the venous sinuses were noted on T2-weighted gradient-echo and T1-weighted spin-echo images. Cerebral digitized subtracted angiogram (DSA) confirmed an occlusion of both transverse and sagittal sinuses but did not reveal any other potential cause of subarachnoid bleeding. The patient was treated with intravenous heparin, with complete radiologic and clinical recovery at follow-up. Results of the etiologic workup were negative.
Case 1. A–B, FLAIR images (9800/152/2300/1) showing hyperintensities sparing the basal cisterns and involving the sulci of the right convexity, compatible with an acute subarachnoid hemorrhage.
C, T1-weighted spin echo image (480/9/1) revealing signal changes within the superior sagittal sinus (arrow).
Case 2
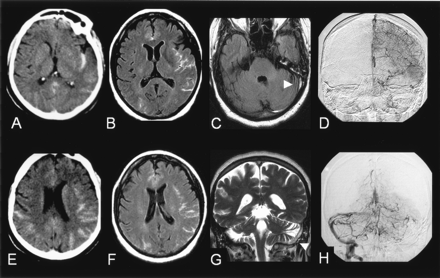
A 55-year-old woman receiving substitutive hormonal treatment had a history of migraine and hypercholesterolemia and was admitted to the hospital for intense, rapidly progressive and pulsatile headaches, neck stiffness, and nausea. These symptoms were followed by a generalized seizure. The administration of acetylsalicylic acid 2 g temporarily relieved the pain. As shown in Figure 2, plain CT scans and FLAIR MR images showed bihemispheric SAH that predominated in the left insular sulci while sparing the basal cisterns. No parenchymal changes were observed. On plain CT scans, a left transverse sinus hyperattenuation was retrospectively observed. A 3D TOF MR angiogram of the intracranial arteries was normal. DSA revealed an occlusion of the sagittal and the left transverse sinus and failed to reveal any cause of SAH. The patient was treated with a low-molecular-weight heparinoid, with rapid clinical improvement and partial recanalization of the thrombosed sinus. No conditions that could result in CVT were found despite an extensive etiologic workup.
Case 2.
A, E, Plain CT scan showing a bihemispheric subarachnoid hemorrhage, predominating in the left insular sulci.
B, F, FLAIR images (9800/152/2300/1) showing hyperintensities within the subarachnoid spaces.
C, FLAIR images (9800/152/2300/1) showing absence of signal changes in the basal cisterns and revealing a hyperintensity within the left transverse sinus (arrow).
G, T2-weighted image (7000/86.3/2) in the frontal plane confirming the presence of a hypersignal within the left transverse and superior sagittal sinuses (arrows), raising the suspicion of sinus thrombosis.
D, H, Venous phase of the digitized subtracted angiogram (A–P views) of the left carotid (D) and the left vertebral (H) arteries confirming the occlusion of the left transverse and superior sagittal sinuses. The right transverse sinus is not seen medially but fills laterally from venous collaterals and drains into the right sigmoid sinus.
Case 3
A 32-year-old woman with no other risk factors for CVT except for the use of oral contraceptive and a recent history of rhinitis was admitted for a partial seizure that was secondarily generalized. She complained of headaches with a sudden onset with vomiting in the previous 3 weeks. A diffuse SAH predominating in the anterior interhemispheric sulci but sparing the basal cisterns was observed on a plain CT scan. MR imaging revealed an occlusion of the sagittal and right transverse venous sinuses, which was confirmed on DSA, without any other potential cause of SAH. Oral contraception was interrupted, and the patient was treated with intravenous heparin, with complete regression of her neurologic signs and headaches. The etiologic workup revealed a hyperhomocystinemia.
Case 4
A 51-year-old woman presented with severe headaches associated with focal neurologic symptoms. The patient, who had a 10-year history of catamenial migraines, was receiving hormonal replacement therapy. Her family history was positive for repeated deep vein thromboses. Two months before her admission, she underwent surgery for a broken ankle and was given preventive anticoagulant therapy because of her prolonged immobilization. Five weeks before her admission, she started complaining of unusual nocturnal headaches. The preventive anticoagulant therapy was withdrawn 1 week before the admission, when the plaster was removed. She was admitted to the hospital because of obnubilation, neck stiffness, recurrent episodes of thunderclap headaches, and head trauma most likely secondary to a sudden loss of consciousness. Several transient episodes of a right neurologic deficit were noted, followed by permanent weakness and ataxia of the right side. A plain CT scan showed a diffuse bilateral acute SAH, sparing the basal cisterns. Findings on FLAIR and T2-weighted gradient-echo MR images confirmed the diagnosis of SAH, which was associated with bilateral nonhemorrhagic edematous areas. These were depicted as parenchymal hyperintensities on diffusion-weighted images with an increased apparent diffusion coefficient. A 3D TOF MR angiogram of the intracranial arteries was normal. CVT was diagnosed on the basis of a contrast-enhanced 3D gradient-echo T1-weighted images that revealed postcontrast filling defects of the sagittal sinus and of the cortical veins draining into it. Hormone therapy was interrupted, and the patient was treated with anticoagulants, with a rapid clinical and radiologic improvement. Results of the etiologic workup for CVT were negative.
Discussion
CVT can be difficult to diagnose because of its large spectrum of clinical manifestations (10, 11). The diagnosis may be further complicated when patients with CVT initially present with acute SAH. Spontaneous SAH is related to a ruptured aneurysm in 85% of cases and to nonaneurysmal perimesencephalic hemorrhage in 10%; the remaining 5% are related to a variety of rare conditions, such as arterial dissection, dural arteriovenous fistula, pituitary apoplexy, and cocaine abuse (12, 13). In an exhaustive review of SAH, CVT was not listed as a cause of SAH (12). This omission is rather surprising because erythrocytes are commonly present in the CSF of patients with CVT (8, 9). In 32 patients with CVT, lumbar puncture showed that 50% had more than 100 erythrocytes per cubic millimeter of CSF. The authors did not mention spontaneous hyperattenuations of the subarachnoid spaces present on CT scans, which were available in 23 patients, although two patients had headaches of sudden onset that simulating an SAH (8). Another report described two patients with more than 100 erythrocytes per cubic millimeter of CSF without a CT correlate (14). To our knowledge, only six patients with CVT associated with acute SAH on CT scans have been reported (1, 3–7), three of whom had an associated hemorrhagic infarct (1, 5, 6). One can speculate that plain CT scan may cause small amounts of subarachnoid blood to be overlooked, especially when the blood is located in the sulci of the cerebral convexity or when larger amounts of extravasated blood are present in the subacute stages.
The exact cause of SAH associated with CVT is unknown. Venous hemorrhagic infarct can be responsible for secondary rupture into subarachnoid spaces and cause SAH (3). Yet none of the patients reported herein had intraparenchymal signs of bleeding on CT or gradient-echo MR imaging. Dural sinus thrombosis with secondary venous hypertension may lead to SAH into the subarachnoid space due to the rupture of fragile, thin-walled cortical veins. Sinus thrombosis may produce dilatation of the cortical veins, which may rupture and bleed into the subarachnoid space and produce an SAH. A similar mechanism has been proposed to explain the presence of extravasated blood confined to the prepontine or interpeduncular cistern in nonaneurysmal perimesencephalic hemorrhage (13).
Three of our four patients (cases 1, 3, and 4) had risk factors for CVT, namely, personal or familial history of deep venous thrombosis (cases 1 and 4); oral contraception, rhinitis, and hyper homocystinemia (case 3); and recent surgery and head trauma (case 4). Of these risk factors, oral contraception and head trauma are also recognized as risk factors for SAH (12, 15). Although head trauma might have been responsible for SAH in case 4, the trauma was most likely the consequence of CVT rather than its cause because clinical signs and symptoms predated the loss of conscience and subsequent trauma by several weeks.
Our observations raise practical issues. Patients with CVT can present with headaches of sudden onset, neck stiffness, and imaging evidence of SAH, simulating a ruptured intracranial aneurysm. The presence of acute SAH of the convexity, especially when basal cisterns are spared, should prompt dedicated vascular imaging of both intracranial arteries and dural sinuses. In most institutions, DSA, which remains the criterion standard for detecting cerebral aneurysm, is still part of the systematic workup for patients with acute SAH. Findings in the venous phase of DSA leads to the diagnosis of CVT, even if it was previously unsuspected. Since noninvasive angiographic techniques are gradually replacing DSA (12) in the workup of patients with acute SAH, the diagnosis of CVT is becoming more challenging. Indeed, in patients with SAH, a CT or MR angiogram focused on arteries of the circle of Willis does not provide adequate imaging of the distal arteries or the venous system during the same imaging session. Therefore, unless CVT is systematically considered in the diagnostic workup of SAH of the cerebral convexity, it could remain undiagnosed when these noninvasive angiographic techniques are used.
Once the diagnosis of CVT is suspected and confirmed on MR imaging or CT, appropriate antithrombotic treatment (i.e., anticoagulant therapy) is considered (1, 5, 11). In our opinion, SAH could be attributed to CVT, provided that formally screening for all other causes of SAH is done. This screening includes a search for distal aneurysms; dural arteriovenous fistulas; and intracranial aneurysms of the circle of Willis, as this has been reported once in association with SAH and CVT (16). Dural arteriovenous fistulas with cortical venous drainage are at relatively high risk of SAH (17) and have been associated with CVT, presumably because of impairment of cerebral venous drainage (18). Therefore, in patients with SAH and CVT, DSA should be considered to rule out other potential causes of rebleeding before anticoagulant therapy is administered.
Conclusion
These cases highlight the fact that SAH may reveal a CVT, which should therefore be considered in the diagnostic workup of SAH, especially when the basal cisterns are not involved. Given the increasing number of patients with headaches who are referred for MR imaging and given the excellent sensitivity of FLAIR sequences for depicting SAH, we are likely to encounter more patients with SAH associated with CVT. Although CT or MR angiograms can be used to diagnose CVT, these noninvasive techniques do not currently replace DSA in ruling out other potential causes of SAH, such as intracranial aneurysm, before the decision is made to introduce anticoagulant therapy.
References
- Received April 9, 2004.
- Accepted after revision June 13, 2004.
- Copyright © American Society of Neuroradiology