Abstract
Summary: We report our observations on MR imaging quality and functionality of a recently introduced intracranial pressure monitoring device. The device was tested at two different field strengths in a pig brain specimen to investigate MR imaging artifacts, probe function during and after MR data acquisition, and device-related temperature changes in the brain tissue. Image reading was not impaired, and probe function, although reduced, was not fully interrupted during data acquisition.
Critically ill neurosurgical patients benefit from continuous intracranial pressure (ICP) monitoring, especially after severe head trauma, subarachnoid hemorrhage, intracerebral hemorrhage, or nontraumatic brain edema (1, 2). MR imaging offers advantages over cranial CT with regard to treatment planning and outcome prediction. It differentiates cerebral structures more reliably, it distinguishes between infarction and edema, and it depicts brain contusions in earlier stages (3–7). Furthermore, brainstem lesions—which may be important in determining the prognosis and outcome—are best detected with MR imaging (8). The monitoring of such critically ill patients is crucial, and none of the commercially available ICP monitoring devices fully meets the definitions of MR safety and compatibility by the US Food and Drug Administration.
The aim of this study was to evaluate the recently introduced Neurovent-P (Rehau AG+CO; Rehau, Germany) ICP monitoring device for use in MR imaging, particularly in terms of image quality, probe function and temperature induction.
Observations
The Neurovent-P ICP device (Rehau AG+CO) (Fig 1), described in detail elsewhere (9), was placed in a straight position with the tip in the white matter of a fresh pig brain specimen at a depth of 2 cm. The specimen was kept in a plastic box filled with physiologic NaCl solution to ensure sufficiently high signal intensity from the probe. Temperature measurements were performed at three time points immediately before, during, and after the acquisition of different MR images. For this we used two clinical MR machines with 1.5- and 0.2-T field strengths and a circularly polarized send-receive head coil (Magnetom Vision and Magnetom OPEN; Siemens, Erlagen, Germany). Two microthermistors (Rehau AG+CO) were positioned—one next to the tip and the other 5 cm away from the tip in the brain parenchyma—to monitor temperature gradients.
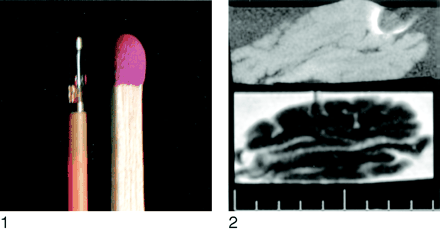
Tip of the Neurovent-P ICP monitoring device (Rehau AG+CO) in comparison with a match.
Fig 2.
Sagittal T1- and T2- weighted images allow good tissue discrimination despite artifact related to the ICP monitoring device.
MR imaging measurements were carried out in a manner suited to neurosurgical demands, and involved sequences for assessing intracerebral or subarachnoid hemorrhage, brain edema, contusion, and infarction. Accordingly, we performed the following sequences in three planes to evaluate the image quality: T2-weighted turbo spin-echo (SE) (TR/TE = 5300/128; turbo factor 23), T2*-weighted gradient-echo (GRE; fast low-angle shot [FLASH] 2D, a TR/TE of 344/52, and a flip angle of 15°), and T1-weighted SE (420/12, three acquisitions). In addition, echo-planar sequences (4295/110) were applied for their high-gradient switching rates and radio-frequency deposition in view of temperature changes. Because of the different field strengths and software releases, the sequences varied for each machine.
Measurements were performed in the same manner to determine the in-magnet functionality of the ICP monitoring device. Functionality was continuously tested during MR imaging data acquisition, while we planned the next examination, and at the end of the examination. The device was thus connected to a patient monitor (Dash 3000, Marquette; Solingen, Germany) via a zero simulator NPS-2 (Rehau AG+CO). The probe was stabilized at a constantly recorded pressure of 2.4 mm Hg. Pressure measurements were recorded at a rate of 10 per second by using a digital data logger (Rehau AG+CO). Data postprocessing was performed by using commercially available software (Excel 2002; Microsoft, Redmond, WA).
Although known for their high-gradient switching rates and artifact induction, transverse T2-weighted TSE images at 1.5 T showed low signal-intensity loss, with a maximum artifact diameter of 6 mm in relation to a 4-mm probe size. Tissue discrimination next to this artifact was not impaired. Similarly oriented T1-weighted images showed excellent tissue discrimination (Fig 2) despite an artifact with a maximum diameter of 15 mm. GRE sequences resolved artifacts larger than those on T2-weighted turbo SE images, with average diameters of 12, 15, and 20 mm in the axial, coronal, and sagittal planes, respectively. Imaging quality was acceptable in the axial plane, satisfactory in the coronal plane, and unsatisfactory in the sagittal plane. The axial echo-planar sequence, which is usually applied for functional imaging at our institution and which is not routinely performed in the acute phase, caused huge artifacts; the resultant image quality was unacceptable.
The T2-weighted images at 0.2 T had good imaging quality regardless of the section orientation. No artifact induction was measurable with 3- and 4-mm artifacts in comparison to the tip size of 4 mm. T1-weighted SE and GRE sequences in any plane showed strong artifact induction, measuring 10 mm on T1-weighted images and 8 mm on GRE images. However, image quality was good.
Temperature measurements taken from the tip of the ICP probe resolved a maximum temperature shift of −0.1°C from a starting point for the 0.2-T scanner and −0.13°C for the 1.5-T system. No relevant temperature gradients were detectable with either system.
We saw no signs of movement or torque when the tip of the probe was exposed to the magnet bore of the 1.5-T system. When the entire probe was exposed to the magnet bore, the plug of the probe was pulled discretely toward the magnet forming an angle of less than 30° relative to its base. The displacement was induced by a force of 6 pounds, less than the total weight of the probe of 12.4 pounds. Gravitation thus has a stronger effect on the fixated probe than the magnetic field.
MR imaging data acquisition affected the measured pressure values to various extents. No changes were detected in pressure values measured inside or outside the machine. The actual pressure was underestimated during data acquisition: The highest values roughly corresponded to the initially measured pressure. Pressure immediately returned to the originally measured value in the intervals between image acquisitions.
Discussion
MR imaging is routinely performed to examine neurosurgical patients. It offers the advantages of soft tissue discrimination, adding information about damage to the blood-brain barrier, early edema formation, and even metabolic (MR spectroscopic) and functional MR imaging analysis. Still, these advantages over CT often cannot be exploited in patients with severe head injury or intracranial hemorrhage. Numerous reports have described the benefit patients gain from continuous monitoring of ICP (1, 2, 10), and the cumulative risk of transporting severely ill patients to the MR imaging unit and disconnecting them from continuous monitoring or removing the pressure measuring device is not justified. Therefore, the demand for MR imaging-compatible continuous ICP monitoring devices is unquestioned. Various attempts have been made to develop MR imaging-safe monitoring and life-support devices for use in anesthesiology as well as MR imaging-safe and artifact-reduced tools for interventional radiology (10–12). For example, the Codman microtransducer (Codman and Shurtleff Inc., Raynham, MA) (to which our group has no access) has been shown to be MR imaging-safe at magnetic fields up to 0.5 T (1). However, this is no longer an environment satisfactory for advanced neuroradiologic imaging, as described before.
Our results show that the newly developed ICP monitoring device, whose clinical safety and reliability were demonstrated in a previous publication (9), is functional in magnetic fields of up to 1.5 T in that it accurately records pressure within the machine and in the intervals between data acquisitions. It continued to do so after data acquisition. The probe itself showed no signs of movement up to 1.5 T, as has been described for the Camino fiberoptic device (Camino Neurocare; San Diego, CA) at 2.0 T (1). Also, no relevant temperature changes exceeding 0.15°C in either direction were detected during data acquisition—either distant from or next to the tip of the device. The possibility of temperature induction applies not only to ferromagnetic materials but also to any other iron material that could act as an antenna.
The main interest was, of course, the imaging quality. If the imaging quality is too poor to deliver additional or more reliable information in patients in neurointensive care, the insufficiencies related to transport and monitoring cannot be justified.
We found adequate imaging quality with both machines when we applied reasonably long and demand-oriented sequences. Imaging results were unacceptable with sequences applied for functional MR imaging; this finding is of interest but of little importance in intensive care patients. T2*- and diffusion-weighted images for visualizing intracranial hemorrhage and ischemia, respectively, yielded acceptable results, at least in one orientation. T1- and T2-weighted images showed excellent tissue discrimination and minimum artifact induction with the device tip in the pig brain specimen.
Conclusion
The good-to-excellent T1- and T2-weighted imaging results obtained in a pig brain specimen definitely encourages us to perform MR imaging without the risk of adverse events. Critically ill neurosurgical patients will benefit from the additional information gained for planning therapy and predicting outcomes. For technical reasons, we have not yet been able to evaluate this ICP device in vivo, and the gap in safety examination prevents us from commenting on its potential in conjunction with neuroexcitation.
References
- Received February 4, 2004.
- Accepted after revision May 20, 2004.
- Copyright © American Society of Neuroradiology