Abstract
Summary: Epistaxis is a common complication in patients with hereditary hemorrhagic telangiectasia. Its treatment is generally aimed at controlling the frequency and severity of nasal hemorrhage and involves surgery, transcatheter embolization, topical treatment, or a combination. Despite this multitude of treatment methods, the long-term prognosis for many patients remains poor. We describe a patient in whom direct intralesional injections of bleomycin successfully palliated severe recurrent epistaxis for almost 2 years without the need for adjuvant therapy.
Hereditary hemorrhagic telangiectasia (HHT) is a familial, multisystemic angiodysplastic disorder characterized by the development of abnormal vessels and vascular malformations throughout the body (1). Arteriovenous malformations may occur in the pulmonary, hepatic, and cerebral circulations, with telangiectasias developing in the oronasal and gastrointestinal mucosa and skin. Epistaxis, resulting from nasal telangiectasias, occurs in more than 90% of patients (1, 2). The multitude of treatment methods that have been used to treat this epistaxis in HHT attests to the difficulty in treating this condition. Methods have included local chemical or electrocauterization; photocoagulation with CO2, argon, or Nd-YAG lasers; transarterial embolization; hormone therapy (eg, topical and systemic estrogen, progesterone, and danazol); treatment with antifibrinolytic drugs (eg, aminocaproic acid, and tranexamic acid); intranasal brachytherapy; and various surgical procedures, such as septodemoplasty, autograft transplantation, vessel ligation, a modified Young procedure, and the use of nasal obturators (2). Novel methods include the use of the harmonic scalpel (3) and the topical application of fibrin glue (4).
Case Report
A 56-year-old man with a diagnosis of HHT had a history of recurrent epistaxis since childhood. For these, he had received multiple treatments, including nasal packing, cauterizations, several blood transfusions, and six surgical and four endovascular embolization procedures. He had also developed a recurrent septal perforation. Given his poor long-term clinical response to the treatments given thus far, we elected to attempt a new technique using direct intralesional injections of bleomycin (Blenoxane; Bristol-Myers-Squibb, Bedfordview, South Africa).
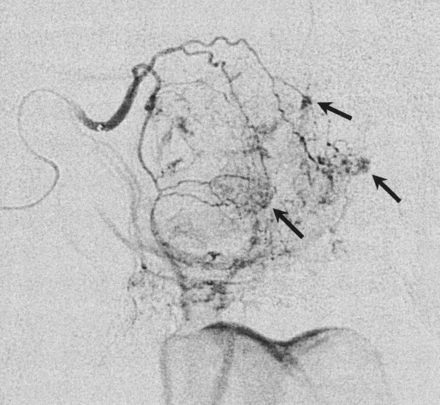
The first bleomycin injection was performed in February 2002 (Fig 1). All were performed as elective procedures with the patient under general anesthesia. One vial (15 U) of bleomycin was dissolved in 15 mL of sterile water. To facilitate handling of the syringe and needle during the injections, a 2-mL syringe was used. Multiple small injections of approximately 0.5 mL each were injected under direct visualization through a 23-gauge needle into the nasal submucosa. These were mainly administered on the right side, particularly around the septal perforation, where most telangiectasias were seen. The injections were performed by placing the needle through the mucosa adjacent to a single telangiectasia or a clustered group of lesions into the submucosa immediately beneath the lesion. The bleomycin mixture was then injected until blanching of the overlying mucosa and the telangiectasia was seen. The blanching was partly due to local pressure by the volume injected as well as possible reflux of the medication into the telangiectasias themselves. After each injection, the needle was removed, and the area was observed for bleeding. Minimal local bleeding was occasionally encountered after each injection; this was easily controlled with local pressure applied by means of a swab for several minutes. A total of 10 mL of the mixture was used during this procedure, although some spillage of the mixture made it difficult to assess the volume actually injected. No nasal packing was inserted afterward. No postprocedural hemorrhage occurred.
Selective right internal maxillary digital subtraction arteriogram shows multiple small, irregular, opacified lesions in the nasal mucosa, in keeping with telangiectasias. Arrows indicate typical injection sites with the needle placed along a lesion and then directed submucosally under the lesion. The bleomycin mixture is then injected until blanching of the overlying mucosa and telangiectasia is seen.
A second elective procedure was performed in June 2002, when 14 U of bleomycin was injected into both nasal cavities. Again, no nasal packing was inserted afterward. Between June 2002 and May 2003, the patient had only occasional, light nosebleeds, none of which required medical intervention.
The patient presented again in May 2003 with mild bleeding, and a third procedure was performed; 7.5 U of bleomycin was injected into the right nasal cavity. Severe intraprocedural bleeding necessitated the application of local pressure and packing of the nasal cavity. Eventually, the bleeding was controlled, and the packs were removed the following day with no further bleeding complications.
A fourth elective procedure was planned for June 2003 but postponed, because the patient had a low hemoglobin level of 10.5 g/dL. This anemia was believed to be chronic, but because of the local bleeding during the previous procedure, we thought that he should first be treated with iron supplementation to improve this anemia before repeating the injection. The patient has since refused any further bleomycin procedure, but telephonic follow-up in November 2003 confirmed that he has had no notable recurrent bleeding since May 2003 and that he is extremely happy with the results of the treatment thus far. He reported far fewer and less severe nose bleeds in the 22 months since his first treatment than at any other time since childhood. For now, we have elected to conduct routine follow-up and reserve further treatments for when the bleeds become severe enough to warrant further intervention.
Discussion
HHT is inherited as an autosomal dominant trait with varying degrees of penetrance and expressivity. Defects in two genes have thus far been identified as being responsible for the induction of the vascular malformations seen in HHT. These genes are endoglin (chromosome 9, HHT-1) and ALK-1 (chromosome 12, HHT-2), both of which encode for vascular endothelial transmembrane receptors of transforming growth factor–β (TGF-β) (1). TGF-β, in turn, plays a role in endothelial cell resolution via activation of the ALK-1 pathway and in endothelial cell activation via activation of the ALK-5 pathway. A reduction of the levels of endoglin in HHT-1 may lead to a decrease in TGF-β levels, affecting both the ALK-1 and ALK-5 pathways. The ALK-5 pathway, however, has a higher sensitivity to the remaining TGF-β than the ALK-1 pathway, thereby preferentially stimulating endothelial cell activation. In HHT-2, reduced ALK-1 proteins leads to preferential relative overactivity of the ALK-5 pathway, again stimulating endothelial cell activation. This activation then stimulates the production of the angiogenic inducer vascular endothelial growth factor (VEGF). Levels of VEGF have been shown to be substantially elevated in patients with HHT (5). Either interruption of TGF-β function or increased levels of VEGF or other related angiogenic factors may thus play a role in the development and growth of the various vascular malformations seen in HHT.
Current treatments in patients with HHT are aimed at controlling symptoms or preventing future complications. One of the most common and most debilitating symptoms in HHT is epistaxis, for which there is also no definitive treatment (2). Our decision to attempt intralesional bleomycin injections in our patient was based on our previous experience and that of others who used bleomycin to treat low-flow venous and lymphatic malformations and hemangiomas (6–10). Given that Mulliken and Glowacki (11) classify telangiectasias as capillary (low-flow) malformations, we believed that these lesions may respond to bleomycin treatment in a similar manner to other low-flow vascular malformations.
Bleomycin is an antimitotic and antimicrobial agent that was discovered in 1965 and later approved by the US Food and Drug Administration in 1975 for therapy against squamous cell carcinomas, testicular cancers, and malignant lymphomas (12). Oikawa et al (13) first reported the antiangiogenic effects of bleomycin in 1990. Licun and Gongjia (14) demonstrated the antiangiogenic effect of pingyangmycing (PYM, bleomycin A5) in chick embryos. On the basis of these findings, they proceeded to treat 14 children with hemangiomas by means of topical injections into the tumors, with complete regression after one or several intralesional PYM injections. Gao et al (15) describes changes occurring after PYM injections into the posterior auricular veins in rabbits, damaging the vascular endothelium immediately after the injection. Endothelial cell and smooth muscle proliferation occurred at 7 days, and finally, vessel occlusion occurred 21 days after injection.
The exact mechanism by which bleomycin produces its antiangiogenic effects is unknown. Both the telangiectasias in HHT and the venous malformations have been linked to elevated levels of VEGF (5), but it is unclear whether bleomycin elicits its effects by suppressing or interfering with the functions or levels of VEGF, TGF, or other angiogenic factors. Another theory suggests that the antiangiogenic activity may be related in part to intracellular free-radical generation (16). Although the exact mechanisms of action are unknown, bleomycin has notable antiangiogenic properties that can be used clinically to treat certain vascular malformations. At the doses used to treat vascular malformations and hemangiomas, none of the major complications related to bleomycin, such as pulmonary fibrosis or Raynaud phenomena, have been observed (6). However, we have seen a number of cases with hyperpigmentation despite the use of relatively low doses (unpublished data).
As we are considering the use of bleomycin as a repeatable treatment for a disease with a propensity to recur, the recommended maximum cumulative dose limit of 400 U (in adults) should probably be observed, although we do not know if this limit can be exceeded over several years without increased risk of pulmonary toxicity. We had originally decided to adhere to our protocol for the treatment of low-flow venous and lymphatic malformations in adults in that the maximal total dose of bleomycin injected per treatment session would not exceed 15 U (6); the actual volumes used were less than this. Some of the mixture was lost when the bleomycin-containing syringe was connected to the needle, when the needle was flushed and displaced, and when external reflux occurred around the needle during the injections. Therefore, the true injected volume was less than the volume “used,” particularly during the first procedure. We also treated only the symptomatic side during the first procedure, expanding the treated area to both sides during the second procedure. We therefore recommend that no more than 7–8 U (7–8 mL) be used in total per side per session.
Local complications that we have encountered during bleomycin treatment for other vascular malformations and hemangiomas include superficial ulceration and cellulitis (6). Knowledge of these potential ischemic complications also tempered our approach with regard to the total volume to be injected initially, particularly in the presence of the recurrent septal perforation. Therefore, the end-point of each procedure was determined by the extent of the area injected (ie, the area of maximum concentration of the telangiectasias with no injection of isolated lesions or normal mucosa beyond). Because of the extreme fragility of the telangiectasias, substantial local bleeding can result from an attempted injection in and around the lesions, as during the third procedure. To minimize this outcome, we have since applied topical cocaine before the injections in a second similar case, with good effect. In this second case, little bleeding was encountered during the procedure. Therefore, the use of vasoconstrictors may be useful before embarking on bleomycin treatment in this setting. Severe intraprocedural bleeding is also a point at which one should consider terminating the injection. If the bleeding cannot be controlled with local pressure or nasal packing alone, urgent embolization or surgical ligation may be required to control it, with further injections planned electively at some later stage. Furthermore, in light of the major bleeding that can occur during the bleomycin injection we recommend that any pre-existing anemia be corrected before a procedure of this sort is done.
Conclusion
Intralesional bleomycin injection adds yet another technique in the treatment of recurrent intractable epistaxis in patients with HHT. The long-term outcome is unknown, and we will closely monitor our patients to assess the results. This particular treatment differs from others described thus far in that it primarily uses the antiangiogenic effect of bleomycin on the causative vascular malformations themselves.
References
- Received February 2, 2004.
- Accepted after revision February 18, 2004.
- Copyright © American Society of Neuroradiology