Abstract
Summary: We report a case of the middle interhemispheric variant of holoprosencephaly (MIH) with noncleavage of the posterior portion of the frontal lobes and the parietal regions in a fetus at 22 weeks’ gestation. To our knowledge, this is the first case of the rare MIH variant to be diagnosed in utero by use of ultrafast MR imaging and one of the few such reports to document gross and microscopic pathologic findings. Neuroimaging results correlated with those of gross and microscopic pathologic specimens obtained from the stillborn child. We conclude that ultrafast MR imaging can accurately distinguish holoprosencephaly subtypes in utero, which may affect counseling of parents.
Although sonography is the mainstay for fetal imaging, MR imaging is often superior in characterizing anomalies of the fetal brain (1, 2). Correctly distinguishing the middle interhemispheric (MIH) variant of holoprosencephaly (HPE) from other forms of HPE and other midline migrational congenital malformations, although difficult with sonography, is possible with ultrafast fetal MR imaging. Ultrafast fetal MR imaging can provide definitive identification and knowledge of the features that differentiate abnormal patterns of brain formation by providing clear and concise delineation of individual brain structures that facilitates diagnosis. This is particularly important, because patients with MIH typically have less severe motor and cognitive disabilities than do those with alobar or semilobar HPE (3). The alobar and semilobar types carry the worst prognoses but are more amenable to reliable prenatal sonographic diagnosis (4). Other midline anomalies, such as agenesis of the corpus callosum (ACC) with interhemispheric cyst, are not infrequently misdiagnosed as HPE, both prenatally and postnatally (5). In general, the long-term outcome in these conditions is significantly better than that of HPE, making the distinction crucial.
Case Reports
A healthy 31-year-old gravida 3, para 2 female patient underwent sonography, performed at 19 6/7 weeks’ gestation, that showed a left-sided congenital diaphragmatic hernia (CDH) and an abnormal fetal brain, described as “asymmetric ventriculomegaly.” Cytogenetic data obtained at subsequent amniocentesis revealed a 46XX karyotype, and the patient was referred to our center for further diagnostic imaging and counseling.
At 22 weeks’ gestation, repeat sonography at our institution confirmed the CDH and suggested a single intracranial ventricle with a dorsal sac and lack of thalamic separation. No cavum septum pellucidum, corpus callosum, or third ventricle could be identified. On the basis of these findings, the diagnosis of lobar HPE was made. In addition, the fetal spine contained a T7–T8 block vertebra and a hemivertebra at T11. The fetal face and extremities were sonographically unremarkable. The intraocular distance was normal for the gestational age.
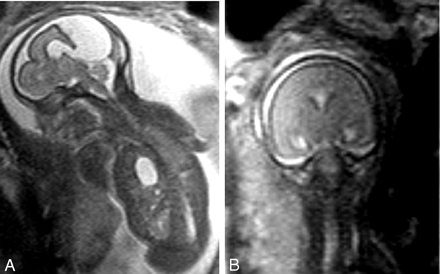
Ultrafast fetal MR imaging of the fetal CNS was performed at 22 weeks’ gestation, the same day that sonography was performed. It disclosed a partially formed anterior interhemispheric fissure with and separation of the frontal poles. The posterior portions of the frontal lobes and the parietal regions were not separated, and the sylvian fissures extended abnormally cephalad to and connected over the calvarial vertex. The deep gray nuclei were separated, and a dorsal cyst was present, which communicated with the ventricular system. No corpus callosum could be appreciated. The constellation of findings visible with fetal MR imaging led to the correct diagnosis of MIH (Fig 1).
Fetal MR images obtained at 22 weeks’ gestation.
A, Sagittal half-Fourier single-shot turbo spin-echo (HASTE) (TR/TE/NA, 1000/95/1) image obtained through the midline reveals the dorsal cyst and cleft formed by communication of the sylvian fissures over the vertex. Herniation of the stomach into the chest is also apparent.
B, Coronal HASTE image obtained through the midportion of the brain depicts the lack of separation of the hemispheres and absent interhemispheric fissure more dorsally.
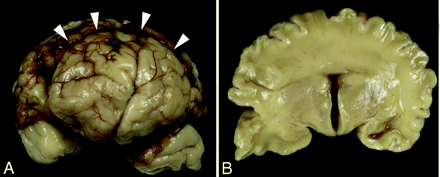
The female fetus was stillborn at 37 weeks. Neuropathologic examination of the brain confirmed all of the neuroimaging findings. There was noncleavage across the dorsal midline with nearly complete obliteration of the third ventricle. In addition, extensive glioneuronal heterotopia was found within the leptomeninges surrounding the entire midbrain and pons and, to a lesser extent, the medulla. Also particularly striking in this case were the microscopic cerebral cortical malformations, which varied in extent according to location. The molecular layer of cortex exhibited an abnormal, undulating appearance extending into layers 2 and 3. The overall cortex was organized in an unusually linear fashion, particularly through layer 4. Layers 5 and 6 were disrupted by numerous collections of myelinated fibers. This abnormal cortex did not fit the pattern of any described dysplasia, but included polymicrogyria. Other features resembled a lissencephaly variant (6) (Fig 2).
Gross specimens obtained from the stillborn female neonate.
A, Frontal view of the brain shows a clear interhemispheric fissure extending to the rostral aspect of the frontal lobes. An abnormal transverse fissure can be seen extending into the dorsal midline of both hemispheres (arrowheads).
B, Coronal section at the level of the third ventricle demonstrates bilaterally symmetric, separated basal forebrain structures (hypothalamus, basal ganglia, and anterior thalamus). In contrast, the cerebral hemispheres are continuous across the dorsal midline with a mass of white matter and cerebral cortex crossing the dorsal midline with no clear corpus callosum.
Discussion
What is distinctive about MIH is the segmental distribution of the anomaly. The smooth anterior-to-posterior progression of severity in classic HPE contrasts with that seen in reported cases of MIH. Imaging studies of MIH characteristically show that the most severely affected portions of the brain are the posterior frontal and parietal convexities, not the base of the prosencephalon in the region of the hypothalamus and subcallosal gyri as seen in classic HPE. The most anterior portions of the frontal lobes and deep gray nuclei are typically widely separated in MIH, also unlike classic HPE. The callosal genu and splenium appear relatively spared in this condition, but the callosal body is absent. The optic apparatus, hypothalamus, and mesencephalon are also spared, and the dorsal cyst, which is thought to be formed by evagination of the floor of the third ventricle due to blockage of CSF flow secondary to thalamic noncleavage, is less common (7–9). The cerebral arterial system is relatively spared, although most patients have an azygous anterior cerebral artery (4, 9).
An additional characteristic feature is the nearly coronal orientation of the sylvian fissures, which are often connected over the vertex of the brain (8–10). This has previously been interpreted as bilateral schizencephaly (10).
Previous reports have shown an association between MIH and a mutation in the ZIC2 gene (9, 11). This gene appears to be significant in neural tube closure and differentiation of the roof plate of the developing embryo. In a murine model, mutation leads to defects of neural tube closure and holoprosencephaly, seemingly from decreased mitosis and increased apoptosis (12). Without the loss of midline cells via these processes, the interhemispheric fissure cannot form. Mutation of ZIC2 has been postulated to result in the MIH morphology (9).
Marcorelles et al (13) reported five cases of MIH associated with monosomy 13q and their pathologic results. Of these five cases, two demonstrated migrational anomalies, one with a diffuse “lack of differentiation of distinct parts of the cortex” and the other with subependymal heterotopia. To our knowledge, only one documented case of postnatal MR of MIH with polymicrogyria appears in the literature; however, the MR findings were not correlated with histopathologic findings (14). Takanashi et al (14) postulated the “possibility of an unknown genetic factor that affects different stages of neuronal development: cleavage of the prosencephalon . . . and neuronal migration and organization.”
A high degree of correlation between the grade of classic HPE and developmental function has recently been reported (3). Clinically, these differences predominately manifest in mobility, upper extremity function, and expressive language. Alobar and more severely affected semilobar subsets have the greatest disability, whereas children with lobar HPE have lesser clinical impairment (15). MIH-affected individuals have difficulties with spasticity, hypotonia, dystonia, and oromotor development affecting speech and feeding, and it is suspected that this is due to the predominate involvement of motor cortex (3). Patients with MIH tend to have better upper extremity control and expressive language skills than do those with classic forms of HPE (3). This may also be due to differences in maturation of cerebral white matter (3, 16, 17).
The hypothalamus and basal ganglia are commonly affected in lobar HPE, and this presents clinically with choreoathetoid movements, endocrinopathies (particularly diabetes insipidus), and dysregulation of core body temperature (15). These features are not seen in MIH because of its more dorsal distribution.
In contradistinction to all forms of HPE, ACC does not display continuous parenchyma across the midline and has an intact interhemispheric fissure. The typical parallel configuration of the lateral ventricles has been well described and should not be confused with the anomalous configuration of the ventricular system of HPE. The enlargement of the massa intermedia that can accompany ACC should not be confused for a lack of thalamic separation. If not diagnosed with routine prenatal sonography, ACC may present as a severe clinical syndrome in infancy or childhood or as a milder condition in young adults. It has also been reported to present as an asymptomatic incidental finding at any age. The most common first sign of ACC is seizure, which may be followed by feeding and postural difficulties. Although many children with ACC will lead normal lives, formal neuropsychological testing reveals subtle differences in higher cortical function compared with that in age-matched control subjects without ACC (18). Treatment is usually guided by symptoms.
Conclusion
Although the prognosis of HPE is generally poor, prenatal differentiation of MIH from the classic forms of HPE by use of ultrafast MR imaging can provide valuable information to guide counseling of parents. Careful attention to the specific imaging features will improve prenatal diagnosis. The HPE sequence is clinically, pathologically, and genetically heterogeneous (7). Understanding the variability among patients will likely assist in the diagnosis, management, and prevention of this developmental anomaly. All of this will be facilitated by early and accurate diagnosis of distinct HPE subtypes. MIH may have specific clinical and genetic implications when identified.
References
- Received June 29, 2003.
- Accepted after revision November 18, 2003.
- Copyright © American Society of Neuroradiology