Abstract
BACKGROUND AND PURPOSE: Although cerebral circulation time (CCT) is one of the main parameters in cerebral blood flow measurements, its clinical significance is controversial. To assess the importance of CCT by using a nondiffusible indicator, we studied the relationship between angiographic CCT and cerebrovascular reserve.
METHODS: Twenty-eight patients, each with a unilateral occlusive lesion in the internal carotid artery or middle cerebral artery, were examined. To assess the CCT, the regional arteriocapillary circulation time (rACCT) was measured by angiography and the ratio of the value on the occlusive side to the value on the contralateral side was calculated as the rACCT ratio. To estimate the cerebrovascular reserve, acetazolamide-challenged single photon emission CT was used. Patients with a decreased cerebrovascular reserve were defined as the “poor reserve” group, and those without a decrease were defined as the “normal reserve” group. The ratio of the radioactivity count on the occlusive side to the count on the contralateral side was calculated as the asymmetry index, and the proportion of the acetazolamide-challenged asymmetry index to the baseline asymmetry index was defined as the regional reactivity index.
RESULTS: The rACCT ratio in the poor reserve group (n = 19) was significantly (P < .001) larger than that in the normal reserve group (n = 9), and a significant correlation (r = −0.83, P < .01) was found between the rACCT ratio and the regional reactivity index.
CONCLUSION: The angiographic CCT and the cerebral vasoreactivity to acetazolamide on single photon emission CT were well correlated, suggesting that measurement of the CCT by using a nondiffusible indicator could be used as an index of cerebrovascular reserve.
Among the various tracers available for the assessment of cerebral hemodynamics, nondiffusible tracers that do not pass through the blood-brain barrier are theoretically simpler than diffusible tracers. Cerebral angiography using nondiffusible indicators have contributed greatly to the assessment of cerebral hemodynamics in patients with cerebrovascular disease (1, 2).
Cerebrovascular reactivity also provides important information for assessing cerebrovascular hemodynamics, and acetazolamide-challenged single photon emission CT (ACZ-challenged SPECT) has been widely used as an index of cerebrovascular reserve (3–7). Acetazolamide (ACZ), a carbonic anhydrase inhibitor, indirectly dilates the cerebrovasculature by increasing carbon dioxide levels in the blood stream (8–12). Under physiological conditions, ACZ increases cerebral blood flow (CBF), but the degree of cerebral vessel dilation is lower in areas of reduced cerebral perfusion pressure because the cerebral vessels are already dilated (13). As a result, ACZ-challenged SPECT increases the contrast in radioactivity levels observed between regions of adequate vascular reserve and those of inadequate reserve.
The aim of this study was to investigate the dynamics of nondiffusible indicators and determine how this information is related to the cerebrovascular reserve by comparing the angiographic cerebral circulation time (CCT) with cerebrovascular reserve measured by ACZ-challenged SPECT.
Methods
Patients Population
Fifty-eight patients who were candidates for either carotid endarterectomy or external carotid-to-internal carotid artery bypass surgery were seen at our hospital (Department of Cardiovascular Medicine, Osaka-Minami National Hospital, Kawachinagano, Japan) between April 1, 1999, and March 31, 2003. All patients had undergone cerebral angiography and ACZ-challenged SPECT in addition to an examination of their general physical condition, cervical sonography, and MR angiography. From the 58 patients, we retrospectively selected 28 who had unilateral occlusion or severe stenosis (≥70% in diameter) of the internal carotid artery or the trunk of the middle cerebral artery (MCA) with minimal or no infarction visible on CT scans or MR images. These patients consisted of 24 men and four women with a mean age of 67 years (range, 47–83 years). Sixteen patients had experienced transient ischemic attack but not cerebral infarction, and the other 12 patients had either an old atherothrombotic cerebral infarction or a lacuna infarction located in the basal ganglia with an infarction size of <1.5 cm (Table 1). Patients with histories of cranial surgery, infratentorial cerebral disease, or acute ischemic stroke and patients with severe illness were excluded from the study.
Patient characteristics, angiographic findings, and results of acetazolamide-challenged single photon emission CT
Technique of Angiography
For all patients, cerebral angiography was performed by using the Seldinger method and a digital subtraction radiographic angiographic system (Advantx LCA; GE Medical Systems, Milwaukee, WI). All patients received mild analgesic medication with an intramuscular injection of hydroxyzine and pentazocine, not general anesthesia, for securing the physiological cerebral circulation. Blood pressure, electrocardiographic readings, and oximeter measurements were monitored during angiographic examination. An auto-injector (Mark V plus; Medrad, Inc., Indianola, PA) was used to inject the following dosages of diopamidol contrast agent (Iopamiron, 300 mgI/mL; Bergkamen, Berlin, Germany): a total of 8 mL into the common carotid artery at a rate of 7 mL/s, a total of 7 mL into the vertebral artery at a rate of 6 mL/s, and a total of 10 mL into the subclavian artery at a rate of 8 mL/s. Satisfactory serial images of bilateral supratentorial and infratentorial circulation were obtained. All angiographic studies included images of the arterial, capillary, and venous phases. The frequencies of the sequences were 0.27 s/frame in the arterial and capillary phases and 0.66 s/frame in the venous phase. All frames were recorded on digital tapes.
Analysis of Circulation Time
The circulation time was determined by the consensus of two authors (S.Y., M.W.) observing a serial display system. Circulation time was estimated by measuring the interval between the moment at which the contrast medium filled the siphon portion of the internal carotid artery or the top of the basilar artery and the moment at which the capillary stain reached its maximum attenuation. This interval was defined as the regional arteriocapillary circulation time (rACCT) and was evaluated in the bilateral MCA regions in each patient. On the occlusive side, the rACCT of all flow pathways (ie, a physiologic flow pathway and collateral pathways) was estimated, and the longest circulation time was defined as the maximum rACCT. On the nonoccluded side, the rACCT of the MCA territories shown by angiography of the common carotid artery was estimated. The ratio of the maximum rACCT in the hemisphere with the occlusive lesion to the rACCT of the contralateral normal side was defined as the rACCT ratio.
Technique of SPECT
SPECT was performed by using a two-headed rotating gamma camera (MULTISPECT2; Siemens Medical Systems, Inc., Iselin, NJ) interfaced with a dedicated computer system. Sixty images were acquired within 20 min by using a 128 × 128 matrix and a low energy high resolution collimator during a 180-degree rotation. The transaxial sections were reconstructed by using filtered backprojection reconstruction with a Butterworth filter; each reconstructed section was corrected for tissue absorption by using Chan’s method (14).
All patients underwent two SPECT sessions: a baseline study and an ACZ-challenged study. The patients were allowed to rest in a quiet, dimly lit room and were IV injected with 166.5 MBq of 123I-N-isopropyl-p-iodoamphetamine (123I-IMP). Baseline images were obtained 15 to 35 min after the injection of the radiotracer (baseline study). Several days later (5–8 days), the patients were placed in the same environment as for the first session and then IV injected with 1 g of ACZ (Diamox; Lederle Lab Division, Pearl River, NY) during a period of 1 min. Ten minutes later, the patients were injected with 166.5 MBq of123I-IMP, and the ACZ-challenged images were obtained in the same manner as in the baseline study.
Qualitative Estimation of Cerebrovascular Reserve
All baseline and ACZ-challenged SPECT scans were scored for relative perfusion abnormalities by using a 10-level color scale. A comparison was then made, based on the consensus of two physicians (S.Y., M.W.), of the relative perfusion changes between the baseline and ACZ studies. Cases with a 10% (one color change) or more reduction in perfusion in the MCA territory on the occlusive side in the ACZ study, compared with the baseline study, were defined as having a “poor reserve,” whereas cases that did not show a 10% reduction in perfusion were defined as having a “normal reserve” (15).
Quantitative Estimation of Cerebrovascular Reserve
Among the 60 reconstructed sections obtained in the baseline SPECT study, the section showing the most prominent contrast in MCA territories was selected and a region of interest was placed over the whole MCA territory on the occlusive side; a horizontally equidistant region of interest was then positioned on the coincided contralateral-mirrored territories, as shown in Figure 1. The ratio of the quantitative radioactivity count obtained from the region of interest on the occlusive side of the MCA territories to the count obtained from the region of interest on the contralateral side was calculated as the asymmetry index (13, 16). For the ACZ-challenged SPECT study, a section at the same level as the one selected in the baseline study was selected, and the asymmetry index was calculated by using the same methods as in the baseline study. The proportion of the ACZ-challenged asymmetry index to the baseline asymmetry index was then defined as the regional reactivity index.
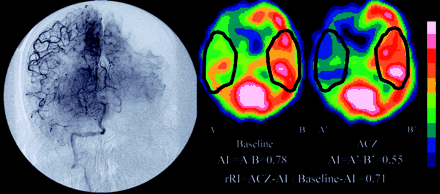
Angiogram and ACZ-challenged SPECT scans of patient 8, who had right internal carotid artery occlusion.
Left, Baseline and ACZ-challenged SPECT scans. Asymmetry index in right MCA territory of baseline scan is 0.78 (A/B) and of ACZ-challenged scan is 0.55 (A′/B′); therefore, regional reactivity index is 0.71 (0.55/0.78).
Right, Angiogram of right vertebral artery. Right MCA territory is perfused via the leptomeningeal artery from the right posterior cerebral artery (PCA). When the left PCA territory is in capillary phase, the right MCA territory is still in arteriole phase. Although the rACCT of left MCA territory is 3.9 s, that of right MCA territory is 8.8 s; therefore, the rACCT ratio is 2.3 (8.8/3.9).
Statistical Analysis
The rACCT data were presented as mean ± SD. Parametric variables were analyzed by using an unpaired t test. Relationships between the rACCT ratio and the regional reactivity index or asymmetry index were evaluated by using linear regression analysis and the Pearson correlation coefficient. P < .05 was considered to indicate a statistically significant difference. All statistical analyses were conducted by using a statistical software package (SPSS for Windows).
Results
Table 1 shows the patients’ characteristics, the angiographic findings, and the results of the ACZ-challenged SPECT examinations. The collateral flow pattern was classified into three patterns: circle of Willis collateral flow, leptomeningeal collateral flow, and external to internal carotid collateral flow. In this study, physiological direct flow via the artery was defined as a “physiological flow pattern.”
Table 2 shows the relationship between the collateral flow pattern and the rACCT in each patient. Although the mean rACCT of the leptomeningeal flow territories was longer than that of the normal arterial territories, the range of the values was very wide (3.4–8.8 s).
Flow pattern and regional arteriocapillary circulation time
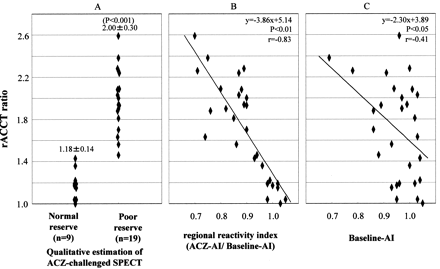
Based on the qualitative evaluation of the ACZ-challenged SPECT scans,19 patients belonged to the poor reserve group and nine belonged to the normal reserve group. The mean rACCT ratio of the poor reserve group was 2.00 ± 0.30, compared with 1.18 ± 0.14 for the normal reserve group. The rACCT ratio of the poor reserve group was significantly larger than that of the normal reserve group (P < .001) (Fig 2A). The quantitative evaluation of the ACZ-challenged SPECT scans showed a significant correlation (r = −0.83, P < .01) between the rACCT ratio and the regional reactivity index (Fig 2B). Although a correlation was found between the rACCT ratio and the asymmetry index in the baseline SPECT study (r = −0.41, P < .05), this correlation was smaller than the one between the rACCT ratio and the regional reactivity index (Fig 2C).
rACCT ratio of poor reserve group is larger than that of normal reserve group.
A, rACCT ratio and qualitative estimation on ACZ-challenged SPECT scan.
B, Correlation of rACCT ratio with regional reactivity index, calculated from ACZ asymmetry index/baseline asymmetry index.
C, Correlation of rACCT with asymmetry index of baseline study.
Discussion
By the 1970s, measurements of the angiographic CCT had contributed greatly to the assessment of cerebral hemodynamics in physiological and morphologic conditions (1–3). To calculate the angiographic CCT, Greitz used arteriovenous CCT: the interval between the maximum filling of the carotid siphon and the maximum filling of the parietal vein (17). A mixture of sodium and methylglucamine diatrzoate (Urographin) was used, and a mean arteriovenous circulation time of 3.43 ± 0.51 s was obtained (18). Greitz explained that although a linear correlation existed between the angiographic CCT and CBF within a fairly wide range in normal patients, this correlation became nonlinear when the CBF was significantly decreased; therefore, he concluded that angiography was more sensitive than the diffusible isotope technique for detecting the decreased flow associated with occlusive vascular disease (19). In this study, we estimated the circulation time by measuring the interval between the moment at which the contrast medium filled the siphon portion of the internal carotid artery or the top of the basilar artery and the moment at which the capillary stain reached its maximum attenuation. This interval was defined as the rACCT. The reason we used the rACCT is as follows. First, concerning the arterial circulation territory, we need to be careful that the venous drainage area in the supratentorial cerebral hemisphere is not consistent with the arterial supplying region. In this study, to detect the region of delayed circulation time with the occlusive artery is most important. However, using the first venous appearance is not sensitive for detecting the delayed circulation; the first venous drainage is mainly influenced by blood drainage from the region with adequate blood supply, not from the region with insufficient blood supply. Therefore, including the venous phase in the inspection of angiographic serial images can lead to underestimation of the regional circulating time. We used the maximum attenuation of capillary phase and found that it was sensitive for detecting delayed circulation. Second, arteriocapillary circulation time is important when considering cerebrovascular reserve, because the regulation of CBF is almost performed by dilation or contraction of arteriocapillary vessels. In addition, it is thought that ACZ produces vasodilation by inhibiting carbonic anhydrase (located mainly in red blood cells) and increasing carbon dioxide level in arteriocapillary vessels (8, 20, 21). Although the CCT has been reported to vary with the measurement points, type of contrast media, method of injection, and age of the population (1), we could indicate a good correlation between the CCT and the cerebrovascular reserve by using the relative value of rACCT: the ratio of the rACCT of the occlusive lesion to the rACCT of the contralateral normal side.
Although we found a correlation between the angiographic CCT and the asymmetry index for the baseline CBF, this correlation was weaker than the correlation between the CCT and the cerebrovascular reserve estimated by using ACZ-challenged SPECT. In a previous study, Gado et al (22) found that angiographic CCT was correlated with CBF and the mean transit time measured by using C15-labeled hemoglobin. Ito et al (23) showed that angiographic CCT was also correlated with CBF and the oxygen ejection fraction on positron emission tomographic scans; they also showed that angiographic CCT was more significantly correlated with the oxygen ejection fraction than with CBF. In our study, we found that the rACCT data measured by using angiography was more significantly correlated with the cerebrovascular reserve than the regional CBF on SPECT scans, indicating that the circulation time can be a more influential index of cerebrovascular reserve than CBF. The reason for this relation can be clarified by considering that CCT reflects mean transit time. The equation mean transit time = cerebral blood volume/CBF shows that mean transit time can be an index of the degree of cerebrovascular dilation or the capacity of cerebrovascular dilation (1, 24, 25). In this study, we confirmed this theory by comparing angiographic CCT and ACZ-challenged SPECT.
Although the materials were focused on the patients with chronic cerebrovascular disease in this study, angiographic circulation time will be useful for evaluating cerebrovascular reserve in cases of acute cerebral ischemia. In cases of acute cerebral infarction, an evaluation of the residual reserve of CBF is very important for determining adaptations to intra-arterial thrombolytic therapy. When thrombolytic therapy is performed in cases with a significantly reduced residual reserve of CBF, the prognosis of the case should be worsened by the occurrence of hemorrhagic transformation (26). Although perfusion MR imaging, perfusion CT, and SPECT studies can be used to estimate CBF in cases of acute cerebral infarction (27–29), these examinations require time to perform and may delay the start of thrombolytic therapy. With additional studies of patients with acute stroke, the critical prolongation in angiographic circulation time could be settled, aiming at early decision for thrombolytic therapy. Because the patient population in our study was comprised of patients who were stable and had chronic disease, it is not certain how the data reported relates to persons with acute ischemic stroke.
Conclusion
The angiographic CCT and the cerebral vasoreactivity to ACZ on SPECT scans of this patient population were well correlated. This suggests that in this patient population, measurement of the CCT by using a nondiffusible indicator could be an index of cerebrovascular reserve.
Acknowledgments
We thank Shigeru Yoneda and members of the Department of Radiology for technical support in performing brain SPECT and cerebral angiography.
References
- Received May 18, 2003.
- Accepted after revision August 4, 2003.
- Copyright © American Society of Neuroradiology