Abstract
Summary: We report the clinical and imaging findings in the cases of two children who initially presented with back pain related to epidural AVF in the cervicothoracic spine. Both lesions were of particular interest because of their exclusive epidural and paraspinal venous drainage and the presence of the prominent venous pouches in the epidural space. Angiography revealed that one was multifocal and of relatively slow flow. We think that these unusual features have important implications for treatment.
Spinal arteriovenous malformations are rare entities that can be classified according to various schemes (1, 2). However, they are commonly divided into three categories according to their location and arterial supply: intramedullary arteriovenous malformations, perimedullary AVF, and dural AVF. All types eventually drain via the coronal venous plexus. The vascular nidus of intramedullary arteriovenous malformations is by definition located within the spinal cord substance itself and fed from the anterior and/or posterior spinal arteries. Intradural perimedullary AVF are characterized by an abnormal direct arteriovenous connection on the surface of the spinal cord without an intervening nidus. Their arterial supply from the anterior and posterior spinal axes is more variable. Dural AVF derive arterial blood from radiculomeningeal branches of segmental spinal arteries, and the fistula is usually located within the dural sleeve of an exiting nerve root. The venous drainage is retrograde toward the spinal cord through the radiculomedullary veins. Our two cases of epidural AVF do not fit into the above classification because the AVF were fed by metameric (segmental) branches and drained exclusively into the epidural and paraspinal venous plexuses. Epidural AVF have been reported in the literature (3–6), but the angioarchitecture in our cases was unique. The arteriovenous shunting in our cases was slow, and pooling of blood occurred within a large epidural venous pouch. To our knowledge, such an entity in young children has not been reported.
Case Reports
Patient 1
A 4-year-old girl presented to an outside hospital with a history of nonradiating sharp upper thoracic back pain, which awoke her from sleep in the early hours of the morning on the day of admission. The pain was relieved by the brief application of a hot water bottle until it recurred and worsened in the afternoon together with the onset of dizziness, bilateral leg pain, and unsteady gait. At the time of admission, the patient was afebrile and her vital signs were within normal limits. Her physical examination was notable for mild tenderness over the C6 spinous process, lethargy, a wide based gait and ataxia, with poor performance on finger-to-nose and heel-to-shin testing. No meningeal signs, abnormalities of the cranial nerves, sensation to light touch, or objective weakness in the extremities were elicited. Her medical history was remarkable only for a resolved recent upper respiratory tract infection, which had been treated with a 1-week course of antibiotics that had been completed 1 week previously, and a tonsillectomy and adenoidectomy procedure that had been performed approximately 2 months before presentation.
Additional testing at the time of initial evaluation included lumbar puncture, which revealed CSF leukocytosis with 9 WBC and a predominance of neutrophils but no xanthochromia. Complete blood count and serum electrolyte panel were normal. The results of CT of the head were normal, as were the results of plain radiography of the cervical spine and whole body nuclear medicine bone scan. The patient was treated with IV administered ceftriaxone and acyclovir, and a provisional differential diagnosis of meningitis or encephalitis/cerebellitis was made. During the next 4 days of her hospitalization, her gait remained abnormal despite improvement in her ataxia. However, her episodic back pain continued to worsen and required institution of a morphine sulfate IV pump with continuous and patient-controlled bolus infusions.
On day 5 of her admission, she was transferred to our institution for further evaluation. The results of repeat CT and MR imaging of the head were normal. However, an MR imaging examination of her spine (Fig 1A and B) revealed a left dorsolateral moderate-sized epidural hematoma extending from C4 to the upper thoracic region. Subtle abnormal vessels were appreciated on this study, with apparent enlargement of the left thyrocervical trunk and epidural venous plexus. These findings prompted spinal angiography (Fig 1C and D), which revealed a slow-flow epidural AVF with a large epidural venous pouch at the C6 and C7 levels. The arterial supply originated from a branch of the left costocervical trunk. The arterial feeder was only mildly enlarged, and no recruitment of adjacent arteries was observed. Multiple smaller epidural AVF were evident adjacent to the largest venous pouch. These were fed by separate branches of the left and right costocervical arteries. The venous drainage of the largest pouch was via the epidural and paraspinal venous plexuses, with no reflux into the intradural compartment and perimedullary veins.
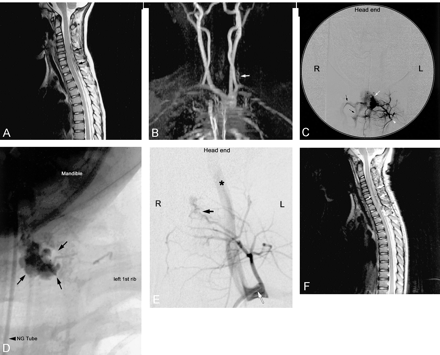
Images from the case of patient 1, a 4-year-old girl who presented to an outside hospital with a history of nonradiating sharp upper thoracic back pain.
A, Sagittal view fast spin-echo T2-weighted MR image. Dorsal epidural hematoma extending between the C4 and T2 levels results in compression of the spinal sac (arrows) but no abnormal intramedullary T2 hyperintensity. Relatively decreased T2 signal intensity in the C3–C6 intervertebral discs may be related to the neighboring epidural AVF and hematoma.
B, Frontal view maximum intensity projection 2D time-of-flight MR angiogram shows an enlarged left thyrocervical trunk (arrow).
C, Posteroanterior projection of a selective injection of the left costocervical artery (arrowhead) shows a fistula into a large epidural venous pouch (white arrow) that drains into the epidural and paravertebral venous plexuses (black arrows).
D, Radiograph shows the cast of the liquid adhesive (arrows) within the venous pouch.
E, Postembolization left costocervical angiogram shows that the dominant fistula is closed but that a separate small epidural fistula (black arrow) remains. The catheter tip is located at the left origin of the left costocervical trunk (white arrow). Reflux of contrast material results in opacification of the left vertebral artery (asterisk). Additional small epidural AVF were revealed when the right costocervical trunk was injected (not shown).
F, Postoperative sagittal view fast spin-echo T2-weighted MR image shows enlargement of cervical dorsal epidural hematoma (arrows), with mild cord compression and new intramedullary T2 hyperintensity (arrowhead).
The patient’s back pain persisted and required narcotics for pain relief. Five days later, the patient underwent transarterial embolization of the epidural spinal AVF (Fig 1E) via superselective catheterization of the feeding artery from the left costocervical trunk. A mixture of N-butyl-cyanoacrylate:lipiodol (14:86) was injected. The embolic material reached the large venous pouch, and the dominant fistula was closed. Several hours after the procedure, the patient developed new weakness in both hands and hyporeflexia and flaccid paraparesis of the legs. Sensation to light touch remained intact. This necessitated emergent decompression of the epidural hematoma with laminotomies from C5–T2. The embolized venous pouch was detected and did not compress the cord. No postoperative improvement in motor function was observed. Postoperative spinal MR imaging (Fig 1F) showed re-accumulation of epidural blood dorsally from C4–T12 and mild spinal cord compression with an intramedullary T2 hyperintensity from C5–T2 that was not present on the original MR images. A second operation, including removal of laminae from C4–T6 and coagulation of enlarged epidural veins at these levels, was performed.
The patient was transferred to a rehabilitation center approximately 7 weeks after her initial presentation. At her follow-up examination 16 months later, she had recovered full strength in her legs with residual weakness of her left hand and urinary retention requiring catheterization.
Patient 2
A 4-year-old boy presented to an outside clinic with complaints of several days of episodic severe upper thoracic back pain with associated muscle spasm lasting from several minutes to 1 hr. The first episode occurred suddenly while he was playing, was not associated with any trauma, and lasted for approximately 10 min. The pertinent details of his physical examination included normal neurologic findings, with no spinal tenderness or deformity. Complete blood count was normal, but sedimentation rate was slightly elevated at 20. The results of subsequent outside whole body bone scan were normal, but CT of the cervicothoracic junction showed a subtle homogeneously enhancing circumferential epidural mass extending between C7 and T3 without cord compression. The child was transferred to our hospital and underwent MR imaging of the spine. Better depiction of the above described epidural mass was seen extending out through several left-sided C6–T2 neuroforamina. No abnormal signal intensity or enhancement of the adjacent vertebral bodies was appreciated. The differential diagnoses offered were lymphoma, leukemia, an organizing hematoma, and metastasis. A bone marrow biopsy was obtained and found to be normal. Follow-up MR imaging (Fig 2A) performed 4 weeks later showed interval growth of the epidural mass but still no cord compression.
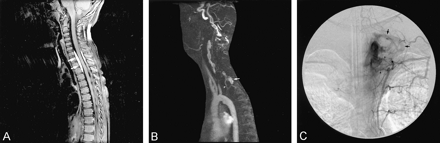
Images from the case of patient 2, a 4-year-old boy who presented to an outside clinic with complaints of several days of episodic severe upper thoracic back pain and associated muscle spasm.
A, Contrast-enhanced sagittal view T1-weighted MR image with fat saturation. A homogenously enhancing circumferential epidural mass can be seen from C7–T3 (arrows). No abnormal intramedullary signal intensity was seen on the T2-weighted MR images (not shown). Associated subtle widening of the anteroposterior diameter of the spinal canal suggests a long-standing vascular malformation.
B, Left oblique lateral view maximum intensity projection from a dynamic contrast-enhanced MR angiogram (technique according to Farb et al [7]). Abnormal enhancing enlarged dural branches can be seen arising from the left supreme intercostal artery (arrowhead), with arteriovenous shunt surgery and enhancement of the large epidural venous sac (arrow).
C, Posteroanterior view projection of selective injection of left supreme intercostal artery. Several large dural arteries feeding AVF (arrowheads) and large epidural venous pouch with drainage via left epidural venous plexus and internal jugular vein (arrows) can be seen.
Surgery was undertaken with C7–T2 laminectomies and biopsy of the epidural mass. This was complicated by intraoperative blood loss requiring transfusion. The results of the pathologic examination indicated no evidence of neoplasm, but an organizing hematoma and thin walled spaces suggested the presence of an underlying vascular abnormality. Repeat MR imaging of the spine included dynamic contrast-enhanced MR angiography (Fig 2B) (7) centered over the proximal great vessels and cervicothoracic spine. This revealed a decrease in size of the epidural mass, which enhanced in the arterial phase of infusion of contrast material. Prominent arterial vessels in the left upper thoracic neuroforamina and possible mild enlargement of the left supreme intercostal artery were detected prospectively. Spinal angiography (Fig 2C) revealed an epidural AVF with dramatic pooling of contrast material in a large venous pouch. Arterial feeders originated from dural branches of the supreme intercostal arteries bilaterally, on the left much more so than on the right. Venous drainage occurred via the left internal jugular and subclavian veins, with no reflux into the intradural perimedullary veins.
The patient was treated conservatively and remained neurologically intact, with no ongoing complaints of neck or back pain. Follow-up MR imaging and MR angiography showed continued decrease in size of the epidural venous pouch and abnormal vessels in the adjacent neuroforamina.
Discussion
Dural AVF comprise the most common type of spinal vascular malformation in the general population (8, 9) but are extremely rare in children. They are seen mainly in the thoracolumbar spine and are typically a disease of the middle-aged and elderly who present with a slowly progressive myelopathy characterized by back pain, radiculopathy, and paraparesis (10, 11). Hemorrhage is very uncommon. The underlying pathophysiology is assumed to be an ischemic myelopathy resulting from chronic venous hypertension (12). Perimedullary direct AVF can present with congestive myelopathy or hemorrhage. However, giant perimedullary fistulae can lead to acute cord compression caused by the enlarged intradural draining vein (13). Intramedullary arteriovenous malformations commonly present in children and young adults. They usually present as a result of intramedullary and/or subarachnoid hemorrhage resulting in acute myelopathy (8, 9). Arterial aneurysms of the enlarged feeding arteries may be responsible for the subarachnoid hemorrhage.
In contrast to the dural and intradural arteriovenous shunts, the symptoms in our two epidural AVF were related to mass effect. In the first case, the mass effect was due to the epidural hematoma. In the second case, the compression was related to the pulsating epidural venous pouch, which probably resulted in mechanical compression of nerve roots and perhaps associated compromise of their venous drainage. The pathophysiology of the acute deterioration in the first patient occurring shortly after embolization was not clear and not predicted. Compression by the thrombosed venous pouch was considered; at surgery, however, the pouch was buried in clot and clearly not responsible for the mass effect. Venous penetration of the large venous pouch by the embolic agent may have compromised the venous outflow from the spinal canal. Compromise of the venous outflow may have resulted in venous congestion in the epidural venous plexus, leading to further swelling in the epidural space or even rebleeding. The presence of the smaller AVF that were not embolized may have accounted for the swelling or rebleeding after the partial treatment. In the presence of multiple separate shunts and a common venous outflow, arterial embolization alone without venous penetration may be the best endovascular treatment strategy.
The management of epidural AVF is not clear because their natural history is not well understood. If patients present with an epidural bleed and no neurologic deficit, treatment should be considered because rebleeding may result in a significant deficit. Embolization alone is ideal for single hole AVF. A high flow, single hole AVF with a single feeding vessel preferably is treated by occlusion with a detachable balloon. Asai et al (5) used a transarterial route and both coils and liquid adhesives with success in an exclusively epidural AVF in an adult. Willinsky et al (4) successfully treated such fistulae in two adults with solely transarterial and combined transarterial and transvenous approaches. If the epidural AVF presents with mass effect that is well tolerated, embolization should be considered when a single hole fistula is present and reachable by using endovascular techniques. In the more complex, multifocal epidural AVF, embolization should be considered as a preoperative adjunct.
The angioarchitecture of our two epidural AVF was unique. Both had irregular, large venous pouches, and both were relatively slow flow shunts. These AVF had not recruited multiple feeding pedicles that are typical of most AVF. Their shunts were unusual because they were characterized by the lack of “sump” effect, which is typical of most pediatric arteriovenous shunts. The irregular epidural pouches may have represented venous pseudoaneurysms that developed after a small rupture of an epidural vein. These atypical features suggest that they were acquired and should be distinguished from the typical congenital arteriovenous shunts observed in pediatric spinal vascular malformations.
The cause of these epidural AVF remains uncertain. Previous reports of epidural AVF in adults have been associated with neurofibromas, neurofibromatosis (14–16), and previous surgery/trauma (14). However, most epidural AVF are spontaneous (3, 4, 17). We suspect that most are acquired because of the lack of significant changes in the adjacent vertebral bodies. However, a congenital or posttraumatic deficiency in epidural venous drainage may predispose a patient to venous thrombosis and subsequent abnormal recanalization by segmental arteries, thus leading to an AVF. This mechanism has been used to explain the origin of typical spinal dural AVF in adults (18–20). A local deficiency in venous anastomoses between the epidural and coronal venous plexuses or a well-functioning “anti-reflux” mechanism in the radicular veins (20) may explain why venous drainage remained exclusively extradural.
Conclusion
We herein present two unusual cases of spinal epidural AVF with exclusive epidural and paraspinal venous drainage, which are also characterized by relatively slow arteriovenous shunt surgery and pooling of blood in large epidural venous sacs. Transarterial embolization of the first case was complicated by enlargement of the epidural hematoma that required surgical decompression. As a result, we think that partial embolization with venous penetration of a multifocal spinal AVF should be avoided. The second case reveals the need for a high degree of suspicion for an AVF presenting as a homogeneously enhancing epidural mass. Furthermore, dynamic contrast-enhanced spinal MR angiography is helpful in the detection of abnormal vessels associated with spinal vascular malformations.
References
- Received December 19, 2002.
- Accepted after revision April 14, 2003.
- Copyright © American Society of Neuroradiology