Abstract
Summary: A 16-year-old male adolescent presenting with acute retro-orbital pain underwent emergent internal carotid occlusion for a giant cavernous aneurysm. Three weeks later, the patient complained of headache and right hemiparesis, which suggested an acute stroke. CT and MR imaging revealed vasogenic brain edema without infarct. The symptoms rapidly resolved with steroid therapy. Follow-up CT showed resolution of the edema. The imaging characteristics, clinical implications, and etiology of vasogenic edema occurring after thrombosis of a giant intracranial aneurysm are discussed.
Giant intracranial aneurysm thrombosis, occurring either spontaneously or after therapeutic occlusion of the parent vessel, may become symptomatic by exerting mass effect on surrounding neural and vascular structures. In rare instances, expansion of the thrombosed aneurysm can eventually lead to an ischemic event in the adjacent brain parenchyma (1). On the other hand, the association of aneurysmal thrombosis with vasogenic brain edema, although exceptional, may wrongly suggest the diagnosis of brain infarction (2, 3). We present a case of symptomatic temporal lobe edema occurring 3 weeks after therapeutic occlusion of the internal carotid artery (ICA) for the treatment of a giant cavernous aneurysm. The imaging characteristics, etiology, and clinical implications of vasogenic edema following aneurysmal thrombosis are discussed.
Case Report
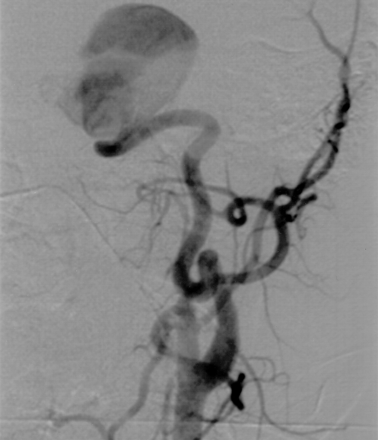
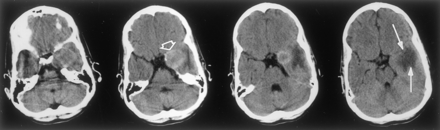
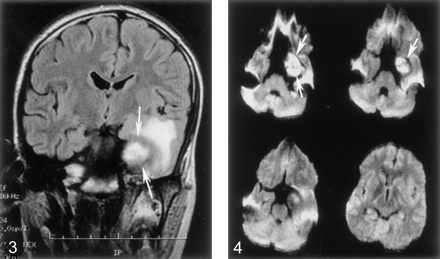
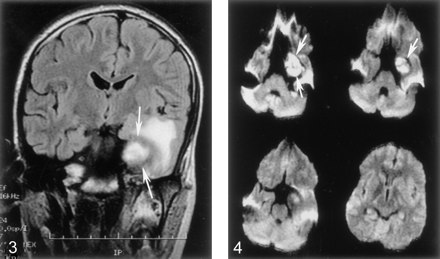
A 16-year-old male adolescent with no remarkable medical history presented to the emergency department with acute left retro-orbital pain. CT scans documented a mass lesion in the left cavernous sinus region; the mass invaded the sphenoid sinus and the middle cranial fossa. Digital subtraction angiography (DSA) revealed a giant aneurysm arising from the cavernous portion of the left ICA, extending medially and superiorly into the middle cranial fossa and inferiorly into the sphenoid sinus (Fig. 1). After successful completion of a carotid occlusion test, therapeutic occlusion of the left ICA with a silicone detachable balloon (Target Therapeutics, Natick, MA) was performed and followed by the proximal delivery of three Guglielmi detachable coils (Target). Control angiograms obtained after ICA occlusion showed no opacification of the aneurysmal cavity despite the presence of brisk collateral flow toward the left cerebral hemisphere via both the anterior and posterior communicating arteries. The immediate follow-up findings were unremarkable. A postprocedural CT scan obtained 2 days after ICA occlusion showed a heterogeneous increase in attenuation within the aneurysmal cavity, consistent with ongoing thrombosis. The patient was discharged home 72 hours after the procedure. He was examined twice during the following week for episodes of mild epistaxis. Each time, the clinical findings were unremarkable. A repeat head CT scan obtained 10 days after the procedure continued to show a thrombosed aneurysmal cavity, less heterogeneous in appearance, but without other interval changes. Three weeks after the intervention, the patient complained of severe headache and a right hemiparesis of sudden onset that suggested a left-sided ischemic event. A left temporal-lobe hypoattenuation was documented by CT (Fig 2). The aneurysmal cavity was hypoattenuating centrally and hyperattenuating peripherally. Its size (3.5 cm) was unchanged when it was compared on the previous CT scans. Emergency brain MR imaging confirmed the presence of a 3.5-cm left parasellar mass consistent with the thrombosed aneurysm. In the left temporal lobe, the white matter immediately surrounding the aneurysm appeared hypointense on T1-weighted images and hyperintense on T2-weighted and fluid-attenuated inversion recovery images (Fig 3). The cortical gray matter was spared. Diffusion-weighted (DW) imaging and apparent diffusion coefficient maps were unremarkable, with no evidence of cytotoxic edema (Fig 4). The imaging characteristics were compatible with vasogenic edema. The patient’s symptoms rapidly regressed with steroid therapy, and the patient was discharged home 3 days later with no residual neurologic signs or symptoms. Except for the thrombosed aneurysmal cavity, findings on a CT scan obtained 2 months later were normal. The patient was neurologically intact at the 12-month follow-up visit.
Digital subtraction angiogram of the left common carotid artery shows a giant cavernous ICA aneurysm, pointing laterally and superiorly.
Axial nonenhanced CT scan of the brain reveals a hyperattenuating thrombosed aneurysm cavity (open arrow) in the left cavernous sinus region. It extends into the middle cranial fossa, with surrounding ill-defined hypoattenuation in the white matter (solid arrows).
Coronal fluid-attenuated inversion recovery (FLAIR) image shows white matter hyperintensity surrounding the thrombosed aneurysmal cavity in the cavernous sinus (arrows).
Axial DW images obtained at the same time as Figure 3 show no restricted diffusion in the white matter surrounding the aneurysmal cavity (arrows). The hyperintensity seen on FLAIR images is compatible with vasogenic rather than cytotoxic edema.
Discussion
Occurring as a consequence of intracranial aneurysm thrombosis, stroke has been described and attributed to different mechanisms. One mechanism is extension of the thrombus from the aneurysmal cavity into the parent vessel, as Brownlee et al described (4). In their report, spontaneous thrombosis extended from an unruptured aneurysm in the anterior communicating artery into the left middle and both anterior cerebral arteries. An alternative mechanism is compression of the parent artery by a large thrombosed aneurysm followed by secondary thrombus formation within the parent vessel. This process has been documented in a case of spontaneous thrombosis of a middle cerebral artery aneurysm (J. Simon et al, unpublished data, 2001). Stroke can also be observed during or after coiling of an endovascular therapeutic aneurysm. This occurrence may be due to release of clot fragments from a partially thrombosed aneurysmal cavity into the cerebral circulation. Although these thromboembolic events can have dramatic consequences, they appear to remain clinically silent in most instances (5). Brain infarction can also occur after therapeutic occlusion of the parent vessel aimed at excluding the aneurysmal cavity from the circulation. A careful angiographic evaluation of the potential collateral supply, as well as the performance of an occlusion test before definitive vessel occlusion, are essential in keeping the risk of such a complication as low as possible.
On the other hand, an ischemic event can complicate an otherwise successful treatment. In this situation, the mechanisms are similar to those associated with spontaneous aneurysm thrombosis; that is, compression of the parent artery by the expanding aneurysm (1) or extension of the thrombus into the lumen of the parent vessel.
Our 16-year-old patient presented with a giant aneurysm of the left ICA that was treated by occluding the parent vessel with a combination of detachable balloon and coils, after a successful balloon occlusion test. The first follow-up CT scan obtained 2 days after the procedure showed a heterogeneous increase in attenuation within the aneurysmal cavity compatible with ongoing thrombosis. The patient was discharged neurologically intact. A second routine follow-up CT study performed 10 days later revealed a decrease in heterogeneity, again consistent with evolving thrombosis of the aneurysm. Three weeks after the intervention, the patient returned, complaining of acute headache and a sudden onset of right hemiparesis. The diagnosis of a left hemispheric stroke was immediately suspected in the emergency department, and it was initially suggested by a large temporal-lobe hypoattenuation depicted on CT scans. However, careful analysis of the CT findings showed sparing of the cortical gray matter; this finding was inconsistent with an ischemic process. The appearance of the aneurysmal cavity was consistent with ongoing thrombosis, and its size was unchanged. Brain MR imaging further clarified the nature of the lesion by confirming the absence of cortical involvement. Normal DW images documented the noncytotoxic origin of the white matter edema and therefore ruled out the diagnosis of acute stroke. On the basis of the imaging findings, steroid therapy was immediately started, with a rapid improvement of the patient’s symptoms. He was discharged home 3 days later with no residual symptoms.
The imaging appearance of the aneurysm after occlusion of the ICA (ie, decreasing intraaneurysmal heterogeneity on the successive CT scans) suggests progressive thrombosis of the aneurysmal cavity; this process was completed at about 3 weeks, when the patient was admitted to the hospital for the episode of acute vasogenic edema. This time course is consistent with the pattern of thrombus formation that Strother et al (6) documented in giant aneurysms after occlusion of the parent vessel. In their study, serial MR images showed an increase in mass effect at the time of thrombus completion, which took place as late as 6 weeks after the procedure. A similar phenomenon was considered in our patient, who presented with acute symptoms 3 weeks after ICA occlusion. However, no enlargement of the aneurysmal cavity was observed, and no other sign of increased mass effect was depicted on the CT or MR images. Massive vasogenic edema has also been associated with spontaneous thrombosis of otherwise asymptomatic giant intracranial aneurysms (7, 8). An increase in the size of the aneurysm after thrombosis with secondary breakdown of autoregulation and swelling of the adjacent brain parenchyma was proposed as a potential mechanism (2). Compromise of venous drainage in the vicinity of the enlarged aneurysmal cavity could also play a role in such instances. In a recent study, the CBF in the area of perianeurysmal vasogenic edema was found to be reduced, with a return to baseline values after the edema resolved. The authors suggested that the enlargement of acutely thrombosing aneurysms result in loss of vasoresponsivity and ischemia (9). Again, these potential mechanisms seem unlikely in our patient, when we consider the absence of aneurysm enlargement and increased mass effect. The development of an inflammatory process in the brain parenchyma surrounding the thrombosed aneurysm has been suggested as an alternate explanation for the edematous reaction. However, no chemical mediators have yet been linked to perianeurysmal vasogenic edema (10), for which the exact mechanism remains unclear at this time.
Conclusion
Symptomatic vasogenic edema can be observed in the brain parenchyma surrounding a thrombosed intracranial aneurysm. Although infrequent, vasogenic edema must be included in the differential diagnosis of strokelike symptoms occurring in the context of spontaneous or therapeutically induced thrombosis of an intracranial aneurysm. MR imaging is important in establishing the definitive nature of the event by demonstrating vasogenic rather than cytotoxic edema on DW images. In our experience, steroid treatment has been proved effective in reducing the extent and clinical impact of the edematous reaction.
References
- Received July 23, 2002.
- Accepted after revision October 21, 2002.
- Copyright © American Society of Neuroradiology