Abstract
Summary: We present a case of an unruptured, large, complex, middle cerebral artery trifurcation aneurysm that was successfully treated by selective occlusion of the neck with a single, newly available UltraSoft coil. The satisfactory initial anatomic result was stable, as demonstrated on a 3-month follow-up arteriogram that indicated complete anatomic cure. The novel UltraSoft coil offers additional possibilities in the endovascular management of difficult-to-treat vascular lesions.
The introduction of detachable platinum coils (Guglielmi detachable coils [GDCs]) for endovascular treatment of cerebral aneurysms has considerably changed the strategy of brain aneurysm treatment worldwide. In particular, for basilar tip aneurysms, endovascular occlusion with GDCs has been accepted as an appropriate therapeutic alternative (1). Aneurysms in other locations such as the middle cerebral artery (MCA) bifurcation, however, are easily accessible at surgery and are therefore still considered primary microsurgical targets (2, 3), Microsurgical clip placement was found to be the safest means of treating unruptured MCA aneurysms (2). Complex vascular architecture, which is typical in many of the MCA aneurysms, is the limiting factor in the feasibility of endovascular techniques (3). The endovascular treatment of larger MCA aneurysms with coils can be especially difficult because, even if complete occlusion can be achieved, the long-term stability remains uncertain. Selective occlusion of the aneurysmal neck, which creates an intrasaccular thrombosis, may be ideal, but it is technically difficult to achieve by using currently available endovascular devices. We report the use of a novel UltraSoft GDC, which enables selective occlusion of an aneurysmal neck, resulting in immediate and complete exclusion of a large complex aneurysm of the MCA trifurcation.
Case Report
Clinical Presentation
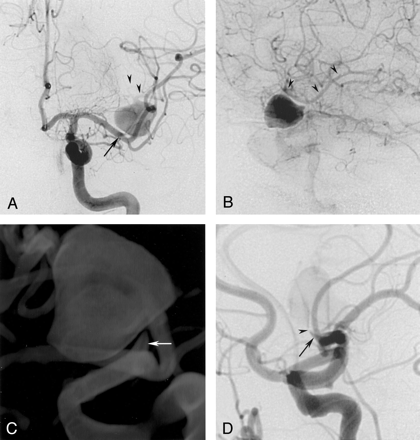
A 41-year-old right-handed woman with no notable medical history was hospitalized for an acute onset of aphasia and numbness in her right hand that lasted for about 5 minutes. At admission, her neurologic findings were unremarkable. CT and MR imaging results were highly suggestive of a large, partially thrombosed aneurysm of the left MCA with no evidence of subarachnoid bleeding. Angiography revealed a saccular aneurysm of the left MCA trifurcation measuring about 14 × 15 mm in diameter. The neck of the aneurysm was difficult to visualize on angiograms because of its small size and narrowed orifice. Three-dimensional rotational angiography revealed that the neck was anteriorly oriented, with a length of about 6 mm tapered toward the dome. Two major MCA branches arising from the dome of the aneurysm showed a minor delay in filling (Fig 1A and B).
Initial left internal carotid artery (ICA) arteriograms, anteroposterior (A) and lateral views (B and D).
A and B, Images show a large aneurysm originating from the MCA trifurcation with a short neck from the distal M1 segment (arrow in A). Two major branches arise from the dome of the aneurysm (arrowheads).
C, 3D image obtained at rotational angiography. The cone-shaped short and broad-based neck is better visualized than on the 2D-DSA images.
D, Lateral-oblique projection obtained during test occlusion. The tip of the microcatheter (arrowhead) cannot be advanced into the aneurysmal dome; it is wedged into the narrowed neck (arrow) that is directed anteriorly and cranially. This placement results in subtotal occlusion of the inflow zone and leads to immediate retrograde filling of the distal MCA territory (not shown).
Given the patient’s history and the complex architecture, endovascular treatment was considered first and started with a selective test occlusion. The occlusion was achieved by blocking the aneurysmal inflow zone with a Tracker Excel 14 (Target Therapeutics/Boston Scientific, Natick, MA) (Fig 1D). Immediate retrograde filling of the involved MCA branches via leptomeningeal collaterals was demonstrated. The occlusion was performed over a period of 15 minutes. The procedure was tolerated by the patient, who had no neurologic symptoms.
Intervention
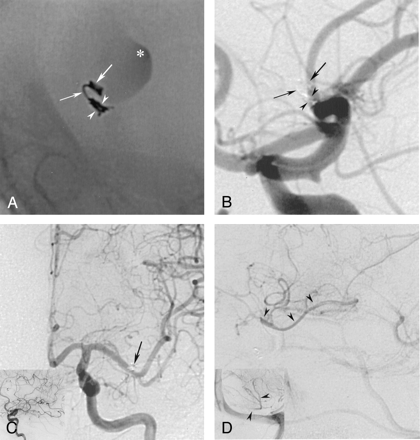
For proximal occlusion of the aneurysm, a Tracker Excel-14 was navigated to the narrowed neck with a wire advanced into the aneurysmal sac. Attempts to navigate the catheter into the aneurysmal sac failed, demonstrating the high degree of luminal narrowing. The tip of the catheter could be advanced only to the very distal end of the stenotic neck. For aneurysmal occlusion, the initial half of a 3/6-cm 3D GDC (Target Therapeutics/Boston Scientific) was introduced into the aneurysmal dome. Then, the catheter was withdrawn slightly, and stabilization of the proximal portion of the coil in the neck was attempted. This maneuver, however, failed because of the size of the coil loops. During these attempts, aneurysmal filling was still visible; it demonstrated that the diameter of the coil itself was too small to achieve complete occlusion of the neck. The same observation was made when we used a regular 0.018-inch coil. To obtain a sufficiently small coil mesh in the aneurysmal neck, we made one more attempt by using a newly available 2/3-cm UltraSoft GDC (Target Therapeutics/Boston Scientific). This coil, which was about twice as soft as a standard GDC-10 Soft coil, was advanced as just described. When we slightly withdrew the catheter, we were able to fold the coil loops in the narrow neck and achieve subtotal occlusion of the aneurysm. Because this packing was still not considered to be stable enough, the procedure was repeated with a longer coil of the same diameter (2/6 cm), which allowed us to place the distal portion (3 cm) in the dome as a sort of anchor (Fig 2A and B). The proximal portion was again folded as tightly as possible in the narrow neck; this procedure resulted in stable positioning. The final arteriogram revealed complete disappearance of the aneurysm and sufficient retrograde filling of the involved MCA branches (Fig 2C and D). The patient woke up with no neurological deficit. She was treated with heparin to maintain an activated partial thromboplastin time of about twice that of the control value for 48 hours. No neurologic symptoms developed in the following days, and the patient was discharged after a week. A 3-month follow-up arteriogram confirmed complete disappearance of the aneurysm and no change in the position or configuration of the coil (Fig 3A and B).
Postembolization arteriograms of the left ICA A, B, and D, lateral views; C, anteroposterior view.
A, Nonsubtracted image. A 2/6-cm UltraSoft coil is placed into the aneurysmal sac with the distal end serving as an anchor (right arrow). The proximal part (3 cm) is placed through the stenosis (left arrow) and then densely folded into the neck. It conforms well to the shape of the neck (arrowheads), with no protrusion into the M1 segment. Asterisk indicates stagnation of contrast material.
B and C, Placement of this single coil (arrows in B) results in the complete disappearance of the aneurysm. In C, the coil loops at the base of the neck and is superimposed onto the lumen of M1 (arrow), but does not actually protrude into it.
D, Late-phase image demonstrate how the involved MCA territory is filled in retrograde fashion by the leptomeningeal collaterals (arrowheads).
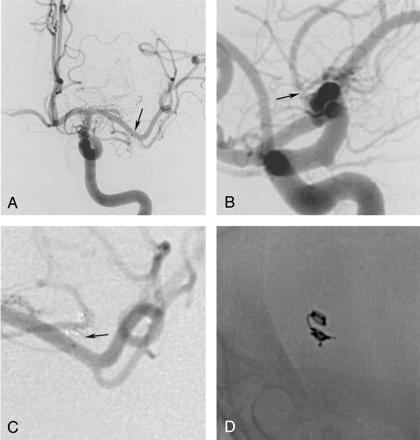
Three-month follow-up arteriogram of the left ICA, anteroposterior (A and C) and lateral views (B and D).
A and B, Images show a patent MCA lumen without a residual neck (arrow) and without coil migration into the aneurysmal sac or protrusion into the parent artery.
C, Magnified view reveals that the coil mesh (arrow) is placed outside the M1-segment.
D, Nonsubtracted view shows an unchanged configuration of the initially placed coil and demonstrates stability of the packing.
Discussion
The high mortality and considerable morbidity rates as a result of complications caused by untreated large or giant aneurysms indicate that treatment is imperative (4, 5). Regardless of the technique used—surgical, endovascular, or a combination—the morphologic features of large or giant aneurysms are persistent challenges in treatment (6, 7). Endovascular treatment of large or giant MCA bifurcation or trifurcation aneurysms can be especially difficult because distal branches arising either from the neck or even the aneurysmal dome are likely to be compromised. Thus, in some cases with unfavorable anatomy, test occlusion and possibly superficial temporal artery-middle cerebral artery bypass may be necessary prior to surgery, aneurysmal coiling, or parent vessel occlusion.
Large or giant aneurysms in this location are surgically treated by means of Hunterian ligation (8) or endovascular occlusion, by using either coils or detachable balloons with or without bypass surgery (6, 7, 9). The reversal of flow may substantially reduce intra-aneurysmal flow and subsequent thrombosis while the distal territory is supplied by the circle of Willis or by leptomeningeal collaterals (7) The Hunterian ligation can be successfully performed at the level of the M1 segment. The procedure allows for progressive occlusion in a patient who is awake. However, it may be associated with ischemic complication due to thromboembolism or inadvertent vessel occlusion (7). As Drake and Peerless (8) emphasized, the treatment of fusiform intracranial aneurysms in which vessels always originate from the aneurysm can be additionally complicated. To achieve thrombotic occlusion of such an aneurysm “the presence of two collateral circulations to prevent infarction, one for the end vessels and another for the perforating vessels that arise from the aneurysm, is required” (8).
Milot et al (9) reported findings in a series of seven patients with giant MCA bifurcation aneurysms who underwent parent-vessel occlusion. A nondetached balloon was used in three patients; a detachable balloon, in one; and coils, in three. The authors achieved complete obliteration of the aneurysm in six patients, with two transient deficits and one permanent ischemic deficit. They observed recanalization in one of the three patients who underwent additional external carotid-internal carotid bypass surgery.
Weill et al (7) reported the successful treatment of two patients with giant MCA trifurcation aneurysms. Both patients were treated with bypass surgery, followed by proximal occlusion of the parent artery with either coils or balloons; one patient had a minor transient neurologic deficit. The authors were able to demonstrate the advantages of endovascular techniques in those aneurysms, the most important of which are the following: 1) avoidance of complications possibly associated with craniotomy and surgical dissection and 2) occlusion of the parent artery at the proper location, which is dependent on the collateral flow pattern demonstrated during the test occlusion (to be performed at the same location).
The specific anatomic configuration in our patient posed a similar technical challenge for the neurosurgeons as well as the neurointerventionalists. In contrast to the cases mentioned previously, we believed that the aneurysm in our patient could have been treated without bypass surgery if a selective test occlusion had been performed and tolerated. We further considered ideal treatment to be not occlusion of the parent vessel, but rather, selective occlusion of the neck with preservation of the M1 segment. The specific technical challenge of this endovascular approach consisted of the very small size and the unfavorable cone-shaped configuration of the neck, which did not provide enough space for a detachable balloon. We further doubted that enough buttressing was present for a stable coil positioning without the use of an adjunctive balloon (remodeling technique) or additional stents. On the other hand, we assumed that the narrow neck could allow the placement of at least one small single coil, which might sufficiently occlude the inflow zone to promote aneurysmal thrombosis. This result, however, was limited by the mechanical properties of the standard GDC, which turned out to be both too large to be completely filled and too stiff to be folded tightly enough into this particular aneurysmal neck. To overcome these limitations, we decided to attempt to fold a newly available UltraSoft coil as tightly as possible. This method proved to be possible and also reproducible by using another coil of the same diameter but a different length (2-mm/3-cm and 2-mm/6-cm coils). Having had some recent positive experiences in treating several small aneurysms with this particular coil, we were nevertheless somewhat surprised that it could even be fitted into such a small and unfavorably shaped vascular space. This placement was apparently possible because of the substantially increased pliability of a smaller platinum wire of 0.0015 inch compared with the 0.00175-inch wire used with regular soft coils. The wire has an ultrasoft nature that allows the coil to yield or bend more easily, and by folding back on itself, it can form an extremely compact mass. Simultaneous achievement of good accommodation to the broad-based neck would not have been possible with any other single-catheter technique. The distal part of the coil was intentionally placed inside the aneurysmal sac as an anchor, which could be further stabilized by the forming thrombotic mass inside the aneurysmal sac, thus holding the coil in place.
Because of the inherent limitations of GDCs, such as coil compaction, and because delayed movement can occur, 3-month follow-up arteriography was performed to detect early untoward changes. This arteriogram, however, confirmed not only complete disappearance of the aneurysm with no residual neck but also the unchanged position and configuration of the coil mesh, which demonstrated its stability. Despite this promising result, longer follow-up and additional experience with similar cases is needed to prove the long-term stability of this type of coil packing.
One may question if the treatment of the aneurysm should have been performed without bypass surgery. Although, to our knowledge, no data on balloon test occlusion of selective MCA or other intracranial branches exist, we believe that the widely used criteria for balloon test occlusion of the internal carotid artery can be applied in intracranial territories as well (10). The fact that the territory involved to be compensated for was only part of the MCA territory may have further contributed to the safety of the test. In addition, this was probably increased by the long-standing stenosis itself, which likely promoted the development of such collaterals in a longer period of time.
Some may also argue that, in view of the patient’s relatively minor clinical symptoms and the narrowed aneurysmal neck (which already obstructed blood flow to some degree), the patient should not have been treated at all, but rather, treated conservatively and followed up with MR imaging. This particular approach was discussed in detail prior to treatment, but the patient eventually refused possible conservative care.
Conclusion
In conclusion, we present a case of a large complex MCA trifurcation aneurysm that was treated with selective occlusion of the neck with a satisfactory initial anatomic result. Follow-up arteriograms obtained at 3 months confirmed the stability of the packing achieved with the UltraSoft coil, which resulted in complete clinical cure. To our knowledge, this particular technique for the treatment of such vascular lesions has not been reported. The UltraSoft coil represents a promising new tool for increasing the packing density during coil embolization of intracranial aneurysms. Although the long-term stability of this type of coil packing in similar cases has yet to be established, the coil may offer additional possibilities in the endovascular management of difficult-to-treat vascular lesions.
References
- Received June 20, 2001.
- Accepted after revision November 9, 2001.
- Copyright © American Society of Neuroradiology