Abstract
BACKGROUND AND PURPOSE: Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) is an arteriopathic syndrome related to a genetic defect on chromosome 19. Characteristic changes in CADASIL can be observed onT2-weighted MR images in the subcortical white matter. The purpose of this study was to measure changes of regional cerebral blood volume (rCBV) with dynamic contrast-enhanced MR imaging and to correlate the changes to disability and cognitive performance.
METHODS: We obtained rCBV measurements of 24 individuals with proven CADASIL on a 1.5-T MR imaging unit. A susceptibility-weighted MR imaging sequence was used for bolus tracking. Principles of the indicator dilution theory were applied to estimate values of absolute rCBV (mL/100 g). Disability was determined by using the Rankin scale, and overall cognitive performance was assessed by using the Mini-Mental State Examination.
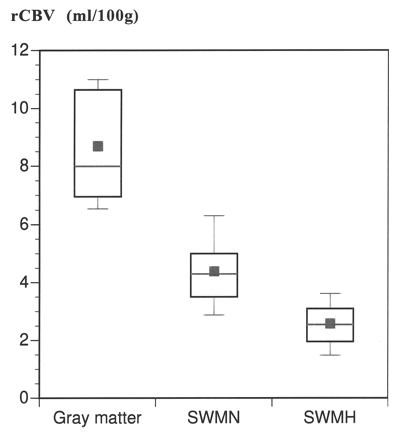
RESULTS: The mean rCBV in the subcortical white matter that was hyperintense on the T2-weighted images (2.7 ± 0.8 mL/100 g) was significantly lower than the rCBV in the white matter that appeared normal on the T2-weighted images (4.4 ± 1.3 mL/100 g) (P < .05). The mean rCBV in the gray matter was within the normal range (8.3 ± 1.7 mL/100 g). Both cognitive impairment and disability negatively correlated with rCBV in the subcortical white matter that was hyperintense (P < .05) but not with rCBV in the normal appearing white matter. rCBV did not correlate with age.
CONCLUSION: rCBV measured in the hyperintense subcortical white matter in individuals with CADASIL was decreased and inversely correlated with disability and cognitive impairment.
Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) has recently been described as a hereditary microangiopathic condition leading to cerebrovascular symptoms in the third to fourth decade of life (1, 2). The affected gene is located on chromosome 19q13 and was recently identified as the Notch3 gene (3–5). CADASIL has been clinically characterized by migraine headaches and the gradual progression of cognitive impairment leading to dementia and increasing disability in mid adulthood (6–8).
MR imaging has been described as the most relevant tool to monitor the cerebral pathology of CADASIL (9, 10). MR imaging is sensitive in displaying both lacunar lesions and diffuse subcortical white matter abnormalities. Using a quantitative approach, we have recently shown that the total volume of MR imaging lesions detectable on T2-weighted images correlates with clinical status (10, 11).
Although CADASIL has been considered a systemic disorder (12), the clinically relevant lesions appear to be located within the brain (6, 13, 14). At macroscopic examinations, two types of lesions are found: diffuse white matter abnormalities sparing the cortical regions and small subcortical infarcts (6, 15). Small vessels exhibited characteristic deposits of granular material (6). These alterations possibly induce severe vessel stenosis, causing prolonged phases of locally limited blood supply.
Therefore, the measurement of hemodynamic parameters may provide an interesting link to the phenotypic expression of the known genetic defect. The determination of regional cerebral blood volume (rCBV) from dynamic susceptibility-enhanced MR imaging measurements is an established tool for the characterization of ischemia (16−18) and enables the differentiation of normal from abnormal perfusion (19). The purpose of our study was to measure rCBV in patients with CADASIL and to correlate the findings to disability and cognitive performance.
Methods
Patients
Twenty-four patients with CADASIL were referred prospectively for MR imaging. Their age ranged between 31 and 68 years (mean age, 52.3 ± 11.7 years). The families involved were identified on the basis of typical clinical symptoms (8), cranial MR imaging showing microangiopathic lesions, and family history compatible with an autosomal dominant trait. In all families, the diagnosis was confirmed by positive skin or muscle biopsy (20). Some clinical details and MR imaging data regarding some of the patients have been previously published (8).
Clinical Assessment
All patients underwent neurologic examination. Their degree of disability was graded according to the Rankin scale (21). Psychometric testing was performed using the Mini-Mental State Examination (MMSE) (22). The MMSE score was then used for further work-up. Dementia was diagnosed if the patient satisfied the Diagnostic and Statistical Manual IV criteria and had an MMSE score of <23 (23). Dementia was also diagnosed in two patients who fulfilled the Diagnostic and Statistical Manual IV criteria but were unable to complete the MMSE. Clinical assessment was conducted and neuropsychologic testing was performed on the same day as the MR imaging.
MR Imaging
All MR imaging was performed on a 1.5-T whole body system (Magnetom Vision; Siemens, Erlangen, Germany), using the standard head coil for RF transmission and detection. After a localizer, T2- and T1-weighted spin-echo sequences with 19 sections were acquired, covering the whole brain (T2: 2300/85, 14/1 [TR/TE/number of excitations]; T1: 530/20/1, 5-mm section thickness, 230-mm field of view). Then, aT2*-weighted gradient-echo sequence (28/.'22/ 1, 10* Rip angle, 3.4 s per section) was used at the level of the basal ganglia for dynamic MR imaging. Dynamic imaging was performed before, during, and after bolus injection of 0.2 mmol/kg contrast material (Magnevist; Schering, Berlin, Germany), injected by a power injector (Spectris; Medrad, Volkach, Germany) at a rate of 5 cc/s.
For quantitative analysis, regions of interest were defined over the brain regions as well as over brain-feeding vessels. The regions of interest in the parenchyma were measured in normal appearing white matter, in frontal subcortical white matter that was hyperintense on T2-weighted images, and in gray matter. The arterial input function necessary to calculate absolute values of rCBV was determined from the middle cerebral artery.
rCBV data were calculated according to the indicator dilution theory (24−26) for the regions of interest and on a pixel-by-pixel basis. The calculations (27) were performed on a separate workstation (SunSparc 10; Sun Microsystems, Mount View, CA). To asses the presence of an intact blood-brain barrier, the signal intensity in normal appearing white matter and in subcortical white matter that was hyperintense on T2-weighted images was measured by regions of interest in the unenhanced and contrast-enhanced T1-weighted sequences of 10 patients.
Statistical Analysis
For comparison of the rCBV values between normal appearing white matter and subcortical white matter that was hyperintense on T2-weighted images, a t test was used. Analysis of variance was conducted to test for correlations between the cerebral blood volume values and the clinical features. For the correlation of age and rCBV, patient age was grouped by decades.
Results
For all 24 patients with CADASIL, a mean cerebral blood volume of 4.4 ± 1.3 mL/l00 g (mean ± SD) was found in white matter that appeared normal on T2-weighted images. In subcortical white matter that was hyperintense on T2-weighted images, the mean rCBV was 2.7 ± 0.8 mL/l00 g (P < .05) (Fig 1). The mean rCBV of gray matter was 8.3 ± 1.7 mL/100 g. Representative MR images of patients with CADASIL are shown in Figures 2 and 3. For some of the patients, lacunardefects isointense to CSF, on both T1- andT2-weighted images, were present at the level of the dynamic susceptibility measurement (Fig 3). For these lacunar defects (n7–10), we measured a rCBV of 0 mL/100 g, as no susceptibility effect during the bolus passage was observed. The blood-brain barrier showed no evidence of leakage; the signal intensity changes after contrast injection were within 5% both in normal appearing white matter and in subcortical white matter that was hyperintense on T2-weighted images.
Mean rCBV of 24 patients with CADASIL, as measured in gray matter and subcortical white matter
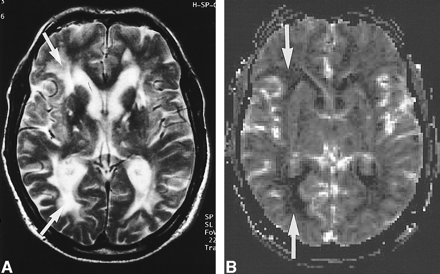
MR imaging findings of a 53-year-old man with CADASIL (MMSE score, not measurable; Rankin score, 4). A, T2-weighted image (2300/85/1). Abnormal increases in the signal intensity in the subcortical white matter are seen in the frontal and occipital white matter (arrows) and in the basal ganglia bilaterally. B, rCBV map of the same section. Decrease of rCBV in the frontal and occipital lobes is detectable (arrows)
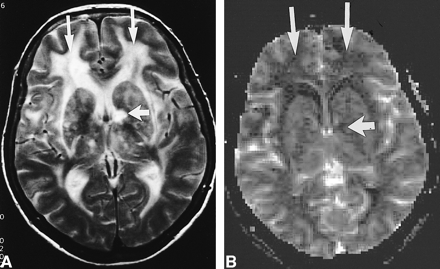
MR imaging findings of a 50-year-old man with CADASIL (MMSE score, 21; Rankin score, 1). A, T2-weighted image (2300/85/1). Lacunar defects (one arrow) and subcortical white matter abnormalities were detected (two arrows). B, rCBV map of the same section. Bilateral reduction of rCBV in the frontal lobes is visible on the rCBV map (two arrows). Lacunar defect visible on the T2-weighted image was also visible (one arrow). rCBV was calculated to be zero
Age Dependency
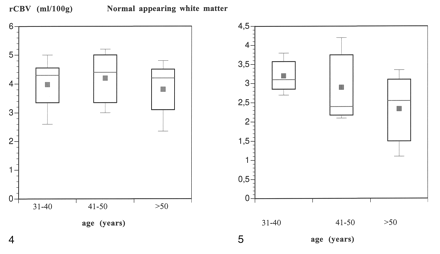
The mean differences in the rCBV of normal appearing white matter for the three age groups were small, ranging from 3.8 to 4.2 mL/l00 g (P > .05) (Fig 4). The rCBV of subcortical white matter that was hyperintense on T2-weighted images was reduced to a mean of 2.4 mL/100 g in the group of patients who were older than 50 years (31−39 years, 3.2 mL/100 g; 41−49 years, 3.2 mL/100 g) (Fig 5). However, statistical analysis showed no significant difference for the rCBV between the age groups in either subcortical white matter that was hyperintense on T2-weighted images (P > .05) or normal appearing white matter (P > .05).
rCBV values of 24 patients with CADASIL in normal appearing white matter as a function of age (error bars denote SD; blue dot denotes mean values).fig 5. rCBV determined in the subcortical CADASIL lesions for each age group
Disability
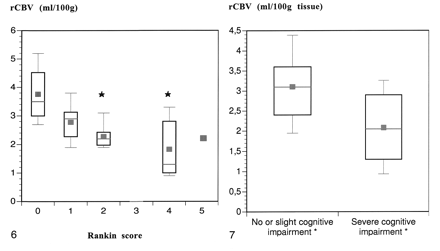
In our group, no patient had a Rankin score of 3 and only one patients had a Rankin score of 5. The relationship between the degree of disability (Rankin) and the rCBV values is presented in Figure 6. A marked trend toward reduced rCBV in patients with higher disability scores was observed. The variance analysis showed a significant difference between the rCBV of subcortical white matter that was hyperintense in patients with a Rankin score of 0 versus 2 and 0 versus 4 (P = .04 and .03, respectively). The correlation between disability and rCBV in the normal appearing white matter was not significant.
Correlation between disability (Rankin score) of patients with CADASIL and rCBV in white matter that appeared abnormal on T2-weighted images. In our group, no patient had a Rankin score of 3. One patient with a Rankin score of 5 had an rCBV of 2.2 mL/100 g. (* = statistically different from Rankin = 0.)fig 7. Correlation between cognitive performance (MMSE score, 22) and the rCBV in white matter that appeared abnormal on T2-weighted images. (*See text for definition of cognitive impairment.)
Cognitive Performance
MMSE scores were available for 22 patients. The relationship between cognitive performance and rCBV values is shown in Figure 7. Cognitive function inversely correlated to rCBV; a significant reduction of rCBV in subcortical white matter that was hyperintense occurred in patients with dementia when compared with patients with no or slightly impaired cognitive performance (P < .05).
Discussion
We found a significant reduction of rCBV within subcortical white matter that was hyperintense on T2-weighted images of patients with symptomatic CADASIL. Compared with previously published results (28), the rCBV values determined in white matter appearing normal on T2-weighted images were within the normal range. rCBV measurements in the lacunar defects showed no measurable cerebral blood volume, regardless of the clinical status of the patient.
Presumably, the reduction of rCBV within subcortical white matter that was hyperintense on T2-weighted images was due to histologic changes caused by granular deposits within or adjacent to the vessel walls (29). Thus, we hypothesize that local accumulation of these deposits gain hemodynamic relevance only within these regions that were hyperintense on T2-weighted images but not in normal appearing white matter.
The correlation between rCBV and clinical parameters indicates that rCBV measurements may be useful for follow-up studies, either to monitor potential treatment protocols or to measure individual progression. A prognostic factor may evolve, considering that the severity of CADASIL in members of the same family is variable (8).
A recent publication reported initial findings of diffusion tensor MR imaging of patients with CADASIL (30). It was found that diffusion-weighted imaging changes were significantly correlated to both disability and cognitive performance (30). The combined use of diffusion-weighted imaging and perfusion (rCBV) measurements is advocated for other conditions, such as acute ischemic stroke (31, 32). Both diffusion and perfusion measurements may play a role in monitoring the neurologic deterioration associated with CADASIL in the future; however, the relative contribution of both measurements in the subcortical lesions in cases of CADASIL is not yet clear.
CADASIL is recognized as a systemic disease, but it presents only with cerebral symptomatology (1, 7, 8, 11, 33). Abnormalities on T2-weighted images of the brain were also more pronounced for patients with severe clinical findings (9). Furthermore, lesion load, measured by T2-weighted sequences, correlated with disability and cognitive deficits (11).
Dynamic susceptibility contrast-enhanced MR imaging has found widespread acceptance for the characterization of tissue microcirculation (28, 34, 35). In the present study, a single-section gradient-echo sequence was used for data acquisition. This sequence was selected because it provides a superior spatial resolution compared with echo-planar sequences (19, 24). However, there are certain limitations associated with our methods: 1) the temporal resolution of the dynamic MR image data set is limited, 2) the data are acquired from one section only, and 3) an age-matched control group was lacking.
Despite these limitations, the rCBV values obtained for normal appearing white matter are not significantly different from those of other groups using similar MR imaging techniques (28, 36, 37) or nuclear medicine techniques (38). Although our values tend to be slightly higher than those of other published reports, we hypothesize that this may have been caused by the use of gradient-echo instead of echo-planar for T2*-weighted imaging. However, the questions of how well a single section measurement does capture the range of lesions and how well this measurement captures the changes in comparison with neurologic and cognitive function remain to be answered in future studies.
Relative measurements of cerebral blood volume based on the indicator dilution theory are valid only if the indicator remains intravascular in the bolus passage. This, in turn, necessitates an intact blood-brain barrier. As no quantitative measurement of the blood-brain barrier has been previously reported for patients with CADASIL, we measured the signal intensity before and after injection of contrast material in 10 patients. The difference in signal intensity was <5%. Considering that these 5% are of the same order of magnitude as the intravasal component in brain tissue, we assumed there was no leakage in the CADASIL lesions. This is in agreement with previous studies, which also did not report contrast material uptake in CADASIL lesions (9, 11).
A recent report described cortical hypoperfusion in patients with CADASIL examined by single photon emission CT (39). In contrast, our results indicate that only the subcortical legions exhibited a reduced blood volume, whereas the rCBV of the cortex was in the range presented in previously published results (40). The reason may be that the two methods measured different results, as single photon emission CT calculated cerebral blood flow (CBF) as opposed to rCBV and the relationship of rCBV and rCBF in CADASIL is currently unknown. The spatial resolution of single photon emission CT is inferior to that of MR imaging, and therefore, single photon emission CT might not allow the differentiation of cortical and subcortical areas in all circumstances. Consequently, the results of the two methods cannot be directly compared. Despite these discrepancies, both studies indicate that CADASIL is a flow-related disorder. Interestingly, signs of hypometabolism and reduced CBF have also been found in association with other conditions, such as leukoaraiosis (41, 42), when measured by fluorine-18-fluorodeoxyglucose positron emission tomography (43, 44) and single photon emission CT (45).
Longitudinal studies of hemodynamic changes in CADASIL and the clinical parameters, such as disability and cognitive performance, are warranted to confirm the impact of rCBV measurements. Although much of the clinical impact of our observation remains as yet uncertain, the observations may be important toward the understanding of the pathophysiology associated with CADASIL.
Conclusion
A significant reduction of rCBV was found in subcortical white matter that was hyperintense on the T2-weighted images of patients with CADASIL but not in normal appearing white matter. The reduction of rCBV significantly correlated with disability and cognitive performance. In addition to the known MR imaging changes, these data shed light on the in vivo hemodynamic situation within CADASIL lesions.
Acknowledgments
The authors thank Juergen Weber, PhD, and Herbert Penzkofer, PhD, for supplying the software and for helpful discussions. Special thanks to Bogdan von Rueckmann, MD, PhD, who helped with the statistics.
Footnotes
1 This study was supported in part by the PR China Fellowship program (to R.H.W.), the Deutsche Forschungsgemeinschaft, and the Friedrich-Baur Foundation (to R.B.).
2 Address reprint requests to Roland Bruening, MD, Institute of Clinical Radiology, Klinikum Grossadern, Marchioninistr. 15, D-81377 München, Germany.
References
- Received May 23, 2000.
- Copyright © American Society of Neuroradiology