Abstract
BACKGROUND AND PURPOSE: Injected air bubbles are a well-accepted cause of stroke during cerebral angiography. We used an in vitro model to determine the frequency of occurrence of air emboli during catheter flushing using conventional hardware and techniques.
METHODS: Two experimental models were used in this study. The first incorporated an in-line bubble trap. Ten members of our angiography section flushed this system in their usual fashion and then with two modifications of the hardware. The trap was inspected after each trial of seven injections and any visible bubble was measured with calipers. The second model used a peristaltic pump along with a transcranial Doppler device to look at the relative number of bubble events with modifications of the flush solution or technique.
RESULTS: The closed-flush set in common usage in our department caused an increase in the number of visible bubbles in the trap as compared with an open basin. Degassing the solution and delaying injection decreased the number of bubble events noted in model 2.
CONCLUSION: Bubble emboli are commonplace during flushing of angiography catheters when using conventional techniques and equipment.
Although the occurrence of procedure-related stroke from cerebral angiography is low, any stroke may be devastating to the patient. Techniques or hardware that further reduce this risk should be constantly evaluated. Many mechanisms for these strokes have been proposed, but one accepted cause is an arterial air embolism introduced during the procedure. This event has come under careful consideration in the past decade, during which sonographic techniques have allowed real-time visualization of intravascular bubble emboli.
During the course of imaging a patient with CT after cerebral angiography, we noticed a small air bubble inside a large aneurysm. The patient had no evident neurologic deficit, but this occurrence suggested to us that air is very likely injected with some frequency in the course of cerebral angiography. As a consequence, we devised two in vitro models that we used to quantify the injected air and to evaluate approaches that might minimize this risk to the patient.
Case Report
A 72-year-old man had an unenhanced CT scan after experiencing dizzy spells. His medical history included a dull frontal headache and decreased vision in his right eye. CT revealed a mass consistent with an aneurysm, which was then confirmed on angiography. Immediately after angiography, another CT study was performed, which revealed a small air bubble inside the aneurysm. The patient had no neurologic deficit from the air embolus and the angiographer reported no difficulties or procedural problems during the examination.
Methods
Model 1
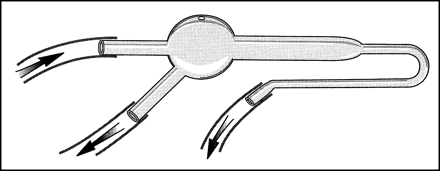
A standard catalog item of glassware was used as a bubble trap. The trap was positioned in line with a flow switch using standard laboratory rubber and tygon tubing (Fig 1). We used 0.9 N sterile saline for the testing. Syringes for flush were filled either from an open basin or a closed-flush set (Merit Medical Systems, Salt Lake City, UT). The glassware and tubing were carefully purged of air using the same solution but injected through a 100-μm filter that effectively trapped any bubbles.
Drawing of the trap shows the inflow and outflow from the device. The outflow on the right was clamped for these tests. A bubble is illustrated in the trap
Ten subjects were recruited from our angiography service. These included three staff radiologists, all of whom regularly perform angiography, two angiography fellows, two residents with angiography experience, and three full-time angiography technologists. The subjects were instructed to perform each trial with the same attention and technique as used in patient care. They were aware that the results would be measured, but the trap was covered and therefore not visible during the trial. After each subject performed seven flushes, the bubble trap was inspected and any visible bubbles were measured with a 3× magnifying lens and calipers. Before the next trial, the system was purged of any air using the filtered solution.
Three experiments were performed with this system. In the first, we used only the standard angiography hardware (ie, plastic 20-mL syringes and a flush kit) employed daily in our department. In the second trial, everything was the same as in the first test, except the subjects were instructed to fill the syringes from an open basin instead of the flush set. In the third trial, the solution attached to the flush set was pressurized to 300 mm Hg and a smaller (10-mL) syringe was used.
Model 2
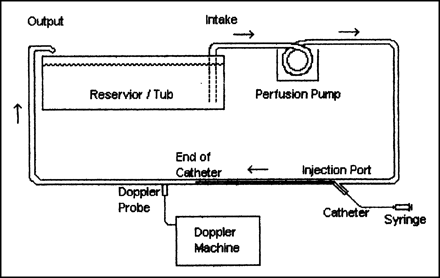
The second experiment was created along the lines of the phantom described by Ginsberg et al (1) in their report of bubbles formed in glass vs plastic syringes. Using flexible rubber tubing and a roller-head perfusion pump, a dynamic system was constructed (Fig 2) that allowed the examination of injected solution with a transcranial Doppler device (EME TC2000, Uberlingen, Germany). An 8-mHz probe was positioned 5 cm downstream from the injection port. The flush solution was injected into a 5F straight angiocatheter positioned so that the catheter tip was near the Doppler probe. A standard IV solution of 5% dextrose in 0.5 N saline was used, and all connections were carefully purged of air.
Schematic shows the arrangement of the pump and Doppler device used to compare the modified flush solutions
Several conditions were tested with this device. First, the solution was injected from the standard closed-flush device immediately after it was drawn up into the syringe from the closed-flush kit. In the second condition, we used solution that was first heated and then put to low vacuum to extract dissolved air but that was still drawn up via the closed-flush set. For the third condition, the untreated solution and closed kit were again used but the fluid was not injected until more than 30 seconds had elapsed from the time it was drawn into the syringe. Three series of 10 injections with each solution were performed.
Results
The results from the bubble trap (model 1) are summarized in the Table.
Measured bubble diameter (mm)
Model 2: Doppler Sonography of Injected Solutions
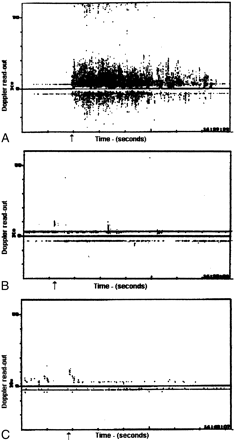
In the first trial with flush injected immediately after being drawn up in the syringe, a consistent pattern of Doppler disturbance was evident on the display, consistent with numerous bubbles in solution (Fig 3). The second trial with deaerated solution appeared very different, with no significant echoes to suggest emboli in the 10 trials. In the third trial, in which the usual flush solution rested for at least 30 seconds in the syringe after being drawn up, the Doppler readout appeared similar to that seen in the second trial.
A–C, This readout from the Doppler device shows the multiple reflections from the bubbles in the flush solution (A). After degassing the solution (B) and allowing the syringe to sit undisturbed for 30 seconds (C), there was an evident reduction in bubble events. A statistical analysis of this data was not available
Applying the data from the Table, we performed a pairwise comparison of the three methods on the outcome measure (bubble generation) using a univariate mixed model analysis of variance (ANOVA: GLM procedure, SAS/STAT 6.12, SAS Institute, Cary, NC) with the method (standard closed flush, open basin, and pressurized flush) serving as the within-subject (repeated) factor and the training level (staff, fellow, resident, and technologist) serving as the between-group factor. As expected, there were no significant main effects or interactions of training level on bubble generation, as all subjects were experienced with angiographic procedures. Therefore, consideration of training level was omitted from further analyses. The principal finding was that the open-basin method significantly reduced bubble generation as compared with the standard closed-flush method (ANOVA: F(1,9) = 9.0; P = .015). The pressurized flush method produced intermediate results that were not significantly different from either the open-basin or standard-flush method.
Discussion
The techniques and equipment used in catheter cerebral angiography have evolved considerably over the past 25 years. Even while the indications for angiography have been dramatically reduced by advances in sonography, CT, and MR imaging, it remains a commonplace and essential diagnostic procedure. The fixed stroke rate is considered to be 0.1%, but this number must be considered in light of the relatively crude neurologic evaluations that patients receive during and after cerebral angiography. Although there have been suggestions in the literature that silent infarcts may occur during angiography (2), this discrepancy was well demonstrated in a study by Bendszus et al (3), who used diffusion-weighted MR imaging to examine 100 patients after cerebral angiography. These investigators found 42 lesions consistent with acute emboli infarcts in 23 patients, none of whom had any signs or symptoms of a neurologic deficit after the procedure. Using a similar approach, Britt et al (4) found no lesions on diffusion-weighted images; however, their study included only 20 patients. These two studies, with disparate results, point out that, currently, the true prevalence and significance of diffusion-weighted imaging findings after angiography are uncertain.
Although outcome studies are reassuring that cerebral angiography is a relatively safe examination, there is a troubling body of research that documents the occurrence of small intraarterial bubbles during cerebral angiography (5, 6). There is little direct evidence that these bubbles are clinically obvious in light of the low overall complication rate of this procedure; however, the studies by Moody et al (7) and Pugsley et al (8) strongly argue that small air bubbles in the inflow lines are the major cause of the neurologic sequelae seen after cardiac bypass procedures. It is reasonable to consider that the size of the bubbles might be important in predicting the effects of bubble emboli. Helps et al (9) studied the effects on animal brains with air emboli of different sizes. They found that, in rabbits, arterial air emboli of 25 μL caused only transient changes in the cortical somatosensory evoked response (SER). A bubble size of 400 μL, however, caused prolonged effects on SER well after all bubbles had cleared and cerebral blood flow had returned to normal. These authors suggest that the effects are, not surprisingly, size-dependent and may explain the variation in severity of effects of diving-related air emboli. All these studies would seem to support any intervention that would reduce or eliminate the occurrence of arterial air emboli during catheter angiography.
Our results indicate that air is very likely injected frequently during catheter flushing and by individuals at all levels of training. While bubbles are commonly seen in the flush syringes, most angiographers assume that maintaining the syringe in the upright position will prevent injection of these bubbles. Our study shows that this technique is not completely effective and that bubbles are injected into the catheter as they are stripped off the wall of the syringe by the plunger. We also found that the results were worse when the subjects used the closed-flush kit, most likely because of the negative pressure needed to fill the syringe and the design of the connector manifold. This negative pressure (which we measured as approximately 150 mm Hg) allows the dissolved air to form bubbles within the solution. This may also explain why Markus et al (6) found fewer intracranial bubbles when the syringes sat for 30 seconds or more. Presumably, this allowed the bubbles to dissolve back into the solution or collect into larger, more readily managed bubbles. Our results with model 2 support the contention by Markus et al (6), since we found fewer bubbles in vitro when the flush solution was allowed to stand. In fact, we saw little difference between this technique and the one in which the syringes were filled from degassed solution.
We hoped that the pressurized flush solution would provide a significant improvement in performance over the closed-flush kit alone. While our subjects' performance was still not as good with this device as with the open basin, for most angiographers and institutions a return to the open basin for angiography is neither feasible nor safe from the perspective of universal precautions. Statistical power may have limited our ability to detect a significant difference between the pressurized flush closed-kit technique. Evaluation of a substantially larger sample of subjects might demonstrate a significant difference in view of the intermediate mean result of the pressurized method obtained in the present study. Although it is possible that a larger sample of subjects might have indicated a significant influence of training level on bubble generation and size, there were no trends along these lines in our data. Our history with the pressurized system to date, however, suggests that it is necessary to have some experience with this system to minimize bubble generation. With practice, it is commonplace to fill the syringes rapidly and with no visible bubbles in the solution. There was a decrease in average bubble size across all operators; and since there is no cost to using the system, we currently use a pressurized flush bag for our diagnostic cerebral examinations.
We did not have the capability to analyze the data obtained from the Doppler device and present these findings only as a graphic display but with obvious visual differences. We did not attempt to calibrate our in vitro system to determine if there were bubbles escaping the trap, and we could find no information in the literature that might provide this value. However, even if there were some spillover of bubbles beyond the trap, it should not affect the findings. Spillover would accentuate the difference in the results between the closed and open systems, since six of the 10 operators had no bubbles after flushing with the open basin and only two had bubbles with the closed kit.
Conclusion
On the basis of our in vitro experiments, we conclude that bubbles are frequently injected during the course of flushing angiographic catheters, particularly when using a closed-flush kit. We look to further studies with advanced MR techniques to determine the effects of modifications of hardware and procedures on patient outcome.
Footnotes
References
- Received May 5, 2000.
- Copyright © American Society of Neuroradiology