Abstract
BACKGROUND AND PURPOSE: Cine phase-contrast (PC) MR imaging is a convenient and effective method for measuring volumetric flow rates in vivo. We attempted to evaluate changes in blood flow in the superior sagittal sinus (SSS) in children and to assess the hypothesis that restricted venous outflow attributable to stenosis of the jugular vein causes hydrocephalus in achondroplasia.
METHODS: Blood flow in the SSS was measured by using cine PC MR imaging with a 1.5-T scanner. After validation, 35 neurologically healthy children as well as eight children with achondroplasia (five with hydrocephalus) and two children with obstructive hydrocephalus were studied. Average flow velocity over the cardiac cycle and volumetric flow rate in the SSS were obtained. The data for healthy children were plotted as a function of age, and reference values were defined by using a five-point smoothing.
RESULTS: In healthy children, flow velocity ranged from 92 to 196 mm/s (mean, 136), and flow rate from 189 to 688 mL/min (mean, 484). The flow rate showed changes statistically related to age. It rapidly increased during the first 2 years and reached a peak by 6 to 8 years of age. The flow velocity showed a similar pattern, but not with significant correlation. In all cases of achondroplasia with hydrocephalus, both flow values were reduced below the reference values minus one standard deviation. In cases of achondroplasia without hydrocephalus, and in obstructive hydrocephalus, the values were not reduced.
CONCLUSION: Blood flow in the SSS reflects brain maturation. Hydrocephalus associated with achondroplasia was found to be closely related to reduced flow in the SSS, which supports the hypothesis that restricted venous outflow causes hydrocephalus in cases of achondroplasia.
Phase-contrast (PC) MR imaging methods exploit the fact that transverse magnetization of spins moving through a magnetic field gradient obtains a velocity-proportional phase shift, allowing for the generation of velocity maps (1, 2). Cine PC MR imaging combines the flow-dependent contrast of PC MR imaging with the ability of cardiac cine imaging to produce images throughout the cardiac cycle. Thus, cine PC MR imaging is a convenient and effective method for measuring volumetric flow rate in vivo (2–6). With this technique, flow in the superior sagittal sinus (SSS) has been measured in healthy adults and under pathologic conditions such as normal-pressure hydrocephalus and benign intracranial hypertension (7). Flow in the SSS, however, which is thought to change with age, has not been fully studied in children during brain development.
Achondroplasia is the most common type of osteochondrodysplasia and the most well known form of dwarfism (8). Recently, mutations in the gene encoding fibroblast growth factor receptor-3 (FGFR3) were revealed in cases of achondroplasia (9). Individuals with achondroplasia have a small skull base but show head enlargement (macrocranium), which is related to hydrocephalus in most cases (10, 11). Mental development in these individuals is within normal range (12). Although the cause of hydrocephalus is still under debate, support exists for the hypothesis that increased pressure in the SSS sondary to restriction of the jugular venous outflow causes hydrocephalus (13–15). If this hypothesis is correct, reduced blood flow in the SSS exists in achondroplastic children with hydrocephalus.
In order to evaluate changes in blood flow in the SSS during brain maturation throughout childhood and to assess whether reduction of venous outflow occurs in cases of achondroplasia accompanied by hydrocephalus, we used cine PC MR imaging to measure blood flow in the SSS in healthy and achondroplastic children.
Methods
Whereas some validation of the quantitative accuracy of cine PC techniques has been reported, additional validation in situations relevant to its envisioned application is desirable (2). To confirm the accuracy of cine PC methods for flow measurement in the SSS, we performed an experiment using a flow phantom. Velocity phase maps were generated at 10 flow rates ranging from 180 to 1720 mL/min by using a simulated electrocardiogram to trigger the sequence with the same parameters as those employed in in vivo studies.
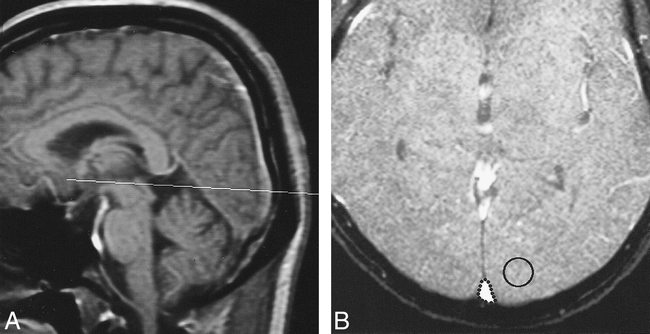
Two in vivo series of flow measurements were performed with cine PC techniques on a 1.5-T MR system (Signa; General Electric, Milwaukee, WI). This slice plane was placed just above the confluens sinuum and perpendicular to the SSS, and 12 evenly distributed data sets per cardiac cycle were acquired with peripheral gating (Fig 1A). Imaging parameters were: 35/12/2 [TR/TE/excitations]; flip angle, 20°, matrix size, 256 × 256; field of view, 20 cm; and slice thickness, 5 mm. First-order flow compensation was used in the read-out direction. Velocity encoding of 60 cm/s was empirically chosen to avoid aliasing. After reconstruction, phase and magnitude images were available. A region of interest (ROI) was manually drawn outlining the SSS on the magnitude images, and a background ROI to correct flow velocity was placed in either occipital lobe, avoiding vessels and CSF (Fig 1B). By superimposing the ROIs on the phase images, flow velocity, averaged in terms of space in the sinus, was obtained as a function of time. Temporally averaged flow velocity over the entire cardiac cycle was then calculated, and multiplying it by the cross-sectional area of the SSS, volumetric flow rate was obtained.
Illustration of flow measurement in the SSS.
A, Slice plane to obtain velocity and magnitude images was placed just above the confluens sinuum and perpendicular to the SSS on a midsagittal T1-weighted image (33/24/2 [TR/TE/excitations]; 20° flip angle).
B, An ROI was manually drawn outlining the SSS on the magnitude images (35/12/2; 20° flip angle). In addition, a background ROI to correct flow velocity was placed in one of the occipital lobes, avoiding vessels and CSF.
The first in vivo series, comprising five healthy volunteers (three men and two women) with an age range of 24 to 40 years were studied to assess the reproducibility of flow measurements. For each subject, the flow was measured twice at an interval of 5 minutes, and the same procedure was repeated 1 week later. All examinations were performed in the normal resting state.
In the second in vivo series, 45 children were studied. They consisted of 35 children (14 male and 21 female) considered to be neurologically healthy, eight children (five with hydrocephalus) diagnosed with achondroplasia clinically and by genetic analysis, and two children with obstructive hydrocephalus (one medulloblastoma and one pilocytic astrocytoma in the cerebellum). The children considered to be neurologically healthy were originally examined to rule out convulsion, trauma, headache, and leukemia and underwent MR imaging to confirm healthy status. The age of the children ranged from 4 months to 16 years. For children older than 8 years (n=29), examinations were performed in the resting state. For children equal to or younger than 8 years (n=16), oral triclofos (60–80 mg/kg) was administered for sedation, and, if insufficient, IV thiopental sodium (2–4 mg/kg) was added. After routine T1- and T2-weighted MR imaging, the flow measurement was performed.
For the statistical study, healthy children were divided into six age groups consisting of approximately the same number (Table 1). The means of flow rate and velocity of the six groups were tested by analysis of variance. Further, the data of healthy children were plotted against age and a five-point smoothing was used to define the running average and standard deviation for each age (the data of the groups with and without sedation were processed independently). Estimated mean values obtained with the five-point smoothing were defined as reference values.
Mean flow values and standard deviation for healthy children in six age groups
Results
The phantom results showed a strong linear correlation (r=0.996; P<.0001) between actual volumetric flow rates and volumetric flow rates determined by velocity phase mapping, and showed agreement with results in the literature (2, 19).
In the first in vivo series, the average flow velocity and flow rate in adult volunteers ranged from 77 to 129 mm/s and from 344 to 402 mL/min, respectively. The acute reproducibility at intervals of 5 minutes revealed a variation of less than 8% in the flow rate, and the first- and second-day flow differed by no more than 10%. Thus, the acute and short-term reproducibility of the flow measurements was confirmed.
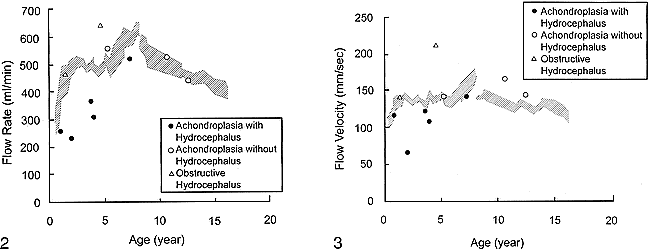
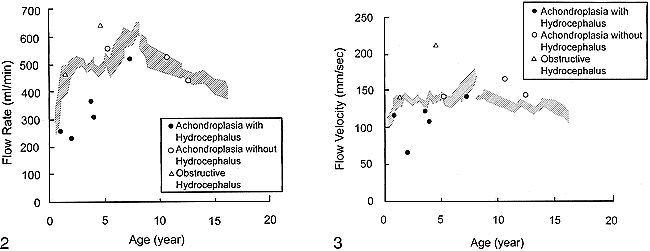
In healthy children, the average flow velocity and flow rate ranged from 92 to 196 mm/s (mean ± SD=136±26) and 189 to 688 mL/min (mean ± SD=484±116), respectively. The intersubject variability was relatively large, but the statistical study of the changes for the six age groups (Table 1) showed a significant effect of age on flow rate (P=.005). Such an effect was not found for flow velocity. The flow rate rapidly increased during the first 2 years, reached a peak by 6 to 8 years of age, and gradually decreased thereafter (Fig 2). The flow velocity showed a similar pattern, but not significantly so (Fig 3).
Flow rate in the SSS plotted against age. The hatched area corresponds to the interval defined by the reference values plus or minus 1 standard deviation, obtained with a five-point smoothing of data of healthy children (the discontinuity at 8 years can be attributed to the fact that the data of groups with and without sedation were processed independently). Flow rate rapidly increased during the first 2 years and reached a peak by 6 to 8 years of age. Thereafter it gradually decreased.
Flow velocity in the SSS plotted against age. The hatched area corresponds to the interval defined by the reference values plus or minus 1 standard deviation, obtained with a five-point smoothing of data of healthy children (the data of the groups with and without sedation were processed independently). Flow velocity showed a similar pattern as flow rate, but was relatively constant
In cases of achondroplasia without hydrocephalus and with obstructive hydrocephalus, the blood flow rate and velocity were above the reference values minus one standard deviation (Figs 2, 3). By contrast, in all five children with achondroplasia and hydrocephalus, both flow values were reduced below the reference values minus one standard deviation (Table 2 and Figs 2, 3). In addition, the degree of reduction was greater for moderate or marked than for mild hydrocephalus.
Measured flow values in achondroplasia and obstructive hydrocephalus and reference values and SD for each age
Discussion
Human brain maturation is incomplete at birth and continues to progress during the first years of life. According to studies with single-photon emission CT or positron emission tomography (PET), CBF and cerebral metabolic rate change significantly with brain maturation. Global CBF increases until 5 or 6 years of age and gradually decreases thereafter, reaching adult levels between 15 and 19 years of age (16). Chugani and colleagues (17) studied the metabolic rates of glucose with PET and found that these rates were low at birth, rapidly rose to adult values at 2 years, continued to rise until approximately 9 years of age, and then declined to reach adult levels late in the second decade of life. The present study of venous flow in the SSS showed a similar pattern, and our results support the view that the flow measurements in the SSS represent an index of global CBF, because the cerebral cortex drains almost exclusively into the SSS (18, 19). Because of this, intersubject differences may arise partly from a certain variety in the drainage pattern of the cerebrum. The flow data as a function of age obtained with our study aid understanding of the correlation between brain development and global CBF, and also provide reference values for different ages that can be used in the presence of pathologic conditions.
Several methodological aspects of our study can be criticized. The first concerns the accuracy of the flow measurements. Some validation of cine PC techniques has been reported, and these in vivo studies do indicate that the PC measurement techniques for pulsatile flow are highly accurate (19, 20). Nevertheless, the accuracy is affected by a number of potential errors inherent to in vivo measurements such as the angle between the vascular axis and the direction of flow sensitivity and definition of the lumen. The angulation of the imaging slice relative to the direction is important, because even 10° off the perpendicular will adversely affect the accuracy. In the present study, a tangential line to the SSS was drawn first and its angle to the horizontal line was obtained, after which the slice plane was positioned at a right angle to the tangential line. In addition, a section thickness of 5 mm was used because it reportedly yields an acceptable level of errors in flow measurements obtained with section planes not absolutely perpendicular to the direction of flow (1). For definition of the lumen, an ROI circumscribing the SSS was manually drawn, instead of simple placement of a circular ROI. Because the flow rate in our study actually differed by no more than 10% after an interval of 1 week, and some changes in physiological conditions were taken into account, the velocity phase mapping method is considered to be satisfactory in terms of accuracy.
The second aspect is the effect of premedication on CBF. CBF is altered by pharmaceutical agents as well as by physiological conditions (18). Pierce et al reported large doses of thiopental can reduce CBF (21). Our study therefore analyzed the effect of thiopental on SSS flow in two groups of healthy children who underwent different types of sedation: one given only triclofos (n=15) and the other given both triclofos and thiopental (n=8). The distribution of the samples was analyzed for equality of variance flowed by the Student's t test for independent samples to compare means. No significant differences in flow rate or velocity were observed between the groups, allowing for the data obtained with and without the use of thiopental to be processed together. The effect of thiopental on cerebral circulation is transient (22, 23), and this may be the result of the flow measurement of the SSS being performed after routine T1- and T2-weighted MR imaging.
Concerning the effect of sleep itself, it is known that global CBF is significantly lower during deep sleep than when awake (24, 25). Our study used either sedation or wakefulness, depending on age, so that a discontinuity between the data in children with and without sedation is currently inevitable. To avoid such discontinuity, further work to determine the exact changes in the SSS flow for the two conditions is needed.
To date, the best noninvasive method to quantify flow velocity in the cerebrovascular system has been transcranial Doppler sonography. Doppler sonographic studies of the SSS or deep venous system, however, can be performed only on infants prior to ossification of the sutures and fontanelles (26). On the other hand, cine PC MR imaging can be performed regardless of age. It also allows for measuring flow volume. Thus, the flow measurements of the SSS obtained with cine PC MR imaging can be used as a robust method for investigating various processes that might affect cerebral venous outflow or global CBF.
We used the reference values defined by the five-point smoothing to evaluate the flow data for children with achondroplasia and for children with obstructive hydrocephalus. In cases of achondroplasia accompanied by hydrocephalus, both blood flow values were reduced below the reference values minus one standard deviation. By contrast, in cases of achondroplasia without hydrocephalus and in obstructive hydrocephalus, the blood flow values were above the reference values minus one standard deviation. Although the number of our patients was too small from which to draw a clear conclusion, and we are not implying statistical significance, the results indicate that hydrocephalus in achondroplasia is closely related to reduced flow in the SSS. In addition, the findings for the two cases of obstructive hydrocephalus may argue against the idea that increased intracranial pressure attributable to hydrocephalus can cause SSS flow reduction.
It has been postulated that hydrocephalus in achondroplasia results from elevated SSS pressure, which in turn results from diminished venous outflow through the small jugular foramen (10, 11, 13–15, 27). This increase in SSS pressure is believed to lead to a reduced pressure gradient across the arachnoid villi, from the subarachnoid spaces to the SSS. When the suture is open in infancy, the resultant increased intracranial pressure results in expansion of the calvarium and ventricles until the pressure normalizes (28, 29). Although our study does not offer direct evidence of increased SSS pressure, the results are consistent with the hypothesis that increased pressure of the SSS attributable to restricted venous outflow causes hydrocephalus in achondroplasia.
Although SSS pressure is determined by CBF and by vascular resistance (30), it is during the first 2 years of life that CBF rapidly increases and the hammering effect of the jugular vein creates the osseous jugular fossa (31). In achondroplasia, a rapid and considerable increase in head circumference related to hydrocephalus has been observed during the 1st year of life (10, 11). All these findings point to the likelihood that dysplasia of the jugular foramen in association with a rapid increase in CBF raises the SSS pressure during infancy and induces hydrocephalus. Although we could not find any reports that achondroplasia shows normal global CBF throughout childhood, it is not inconceivable that normal brain maturation plays a role in the etiology of hydrocephalus in achondroplasia.
Conclusion
Measurements of blood flow in the SSS can represent an index of global CBF and provide an insight into the relationship between brain development and changes in global CBF. Hydrocephalus in achondroplasia is closely related to reduced blood flow in the SSS, which supports the hypothesis that restricted venous outflow attributable to stenosis of the jugular vein causes hydrocephalus in achondroplasia. It is likely that dysplasia of the jugular foramen in association with a rapid increase in global CBF raises SSS pressure during infancy and induces hydrocephalus.
Acknowledgments
We are grateful to Drs. Masahiro Higashi and Yoshitetsu Ohshiro for their excellent and patient assistance in the preparation of this manuscript.
Footnotes
↵1 Address reprint requests to Norio Hirabuki, Department of Radiology, Osaka University Medical School, 2-2 Yamadaoka, Suita City, Osaka 565-0871, Japan.
References
- Received June 14, 1999.
- Accepted after revision March 1, 2000.
- Copyright © American Society of Neuroradiology