Abstract
Summary: Eosinophilic meningoencephalitis is prevalent and widely distributed in Thailand, especially in the northeastern and central parts of the country. Angiostrongylus cantonensis is one of the causative agents of fatal eosinophilic meningoencephalitis. The nematodes produce extensive tissue damage by moving through the brain and inducing an inflammatory reaction. We report the clinical features and the findings revealed by MR imaging and MR spectroscopy in six patients with eosinophilic meningoencephalitis. The clinical presentation included severe headache, clouded consciousness, and meningeal irritation. Abnormal findings on MR images included prominence of the Virchow-Robin spaces, subcortical enhancing lesions, and abnormal high T2 signal lesions in the periventricular regions. Proton brain MR spectroscopy was performed in three patients and was abnormal in one severe case, showing decreased choline in a lesion. Small hemorrhagic tracts were found in one case. Lesions thought to be due to microcavities and migratory tracts were found in only one case. We believe the MR imaging and MR spectroscopy findings are of diagnostic value and helpful in understanding the pathogenetic mechanisms of the disease.
Angiostrongylus cantonensis is a nematode parasite that inhabits the pulmonary arteries and heart of rodents. Infective larval stages are also found in certain snails and monitor lizards in Thailand (1, 2). The organism was first recovered from the CSF of a Japanese patient who died of eosinophilic meningoencephalitis in Taiwan in 1944 (3). In 1962, Rosen and coworkers (4, 5) reported two fatal cases of eosinophilic meningoencephalitis in Hawaii. Another case in which the parasite was found in the brain was reported from Vietnam in 1965 by Jindrak and Alicata (6). Living A. cantonensis was also recovered from the anterior chamber of the eye in three patients in Thailand (7–9).
Epidemiologic observations in 484 typical cases of eosinophilic meningitis caused by A. cantonensis were reported from Thailand by Punyagupta et al in 1970 (10). Most of the patients had eaten raw fresh-water snails, especially Pila sp, which act as intermediate hosts for the parasite (10–12). In 1990, five patients with eosinophilic meningitis were admitted to a teaching hospital in Bangkok. One patient died, another was paralyzed, and three recovered. An autopsy revealed many fifth-stage larvae of A. cantonensis in the brain of the fatal case. All the patients had eaten raw or partially cooked monitor lizard (Varanus bengalensis) before experiencing symptoms (13, 14). Similarly, at Khon Kaen University, among 22 patients who were seen after having eaten monitor lizard, five became ill with eosinophilic meningitis (15). Autopsies carried out in fatal cases of eosinophilic meningoencephalitis have revealed areas of disrupted brain tissue, a massive response by eosinophils to the dead parasites and tracks of penetration (16).
We present the MR findings in six patients (including the MR spectroscopy findings in three) with eosinophilic meningoencephalitis caused by A. cantonensis.
Methods
Six patients who presented with a clinical and laboratory-confirmed diagnosis of eosinophilic meningoencephalitis underwent MR imaging and MR spectroscopy on a 1.5-T system. The technique included fast spin-echo T2-weighted (3500–5000/80–135 [TR/TE]) sequences and pre- and postcontrast T1-weighted (500–600/9) studies in sagittal, axial, and coronal planes. Additional gradient-echo images in the coronal plane were obtained in one case (640/25; flip angle, 20°). A single point-resolved spectroscopy technique (2000/35) was used in three patients with a voxel size of 2 × 2 × 2 cm3 placed on a white matter lesion. The data analysis was performed with commercial software.
Case Reports
Case 1
A 32-year-old Thai man who had eaten raw monitor lizard meat and liver was admitted 2 weeks later to Srinagarind Hospital. After the meal, he had vomited and experienced a mild headache, which gradually increased in severity. At physical examination, he was afebrile but stuporous and had stiffness of the neck, papilledema, and generalized hyperreflexia. There was no muscle weakness. CSF analysis revealed high pressure, a white blood cell count of 353 cell/mm3 (eosinophils, 28%; lymphocytes, 70%; neutrophils, 2%), a protein level of 95 mg/dL, and a CSF/blood sugar ratio of 51%. A complete blood count showed leucocytosis with 18% eosinophils.
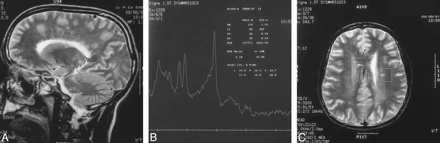
A T2-weighted MR examination of the brain revealed multiple, abnormal, fuzzy, hyperintense signals involving the deep periventricular white matter in the left frontoparietal region and centrum semiovale (Fig 1A). Multiple small areas of enhancing subcortical lesions in the left parietal lobe and pons were also noted. There was mild dilatation of the perivascular subarachnoid spaces. Additional MR spectroscopy revealed a decreased level of choline (with a choline to creatine ratio of 0.7) in the left centrum semiovale (Fig 1B and C).
A, 32-year-old man. Sagittal T2-weighted MR image (3500/80/3) reveals areas of fuzzy hyperintense signal in the left frontoparietal region and centrum semiovale.
B and C, MR spectrum (B) reveals decreased choline (choline/creatine ratio, 0.70) in the left centrum semiovale (voxel indicated by box in C).
Case 2
A 45-year-old man was referred from another hospital with deteriorating consciousness over the course of 1 week. Six weeks before admission, he and his friends had eaten raw monitor lizard meat and liver. After the meal he had vomited and he later experienced a mild headache in the occipital region. The headache became severe and persisted until admission. On admission, he was stuporous but his vital signs were stable. He had neck stiffness and spasticity of the right upper extremity. A lumbar puncture revealed a pressure of 330 mm H20 and a white blood cell count of 134 cells/mm3 (eosinophils, 46%; neutrophils, 3%; lymphocytes, 51%).
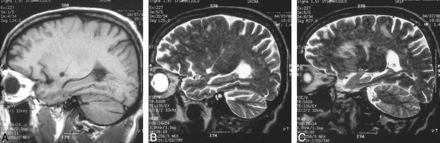
An MR examination of the brain showed scattered areas of abnormal signal intensity in the deep white matter of the periventricular, corona radiata, and subcortical areas of the frontoparietooccipital lobes bilaterally, suggesting gliosis or worm migratory tracks. There was a visually impressive, long, continuous track area with a small hole in the region of the left putamen (well delineated in the sagittal plane) (Fig 2A and B). Areas of hyperintense white matter foci varied in size and shape, with linear, nodular, and patchy configurations (Fig 2C). Mildly dilated perivascular subarachnoid spaces were also noted.
A and B, 45-year-old man. Sagittal T1-weighted (600/9/2) (A) and T2-weighted (5000/135/3) (B) MR images show a long track with a cavity in the left putamen (low signal in A and high signal in B).
C, Sagittal T2-weighted image (5000/135/3) shows abnormally high signal in periventricular, linear, and fuzzy nodular lesions.
Case 3
Two weeks before admission, this 64-year-old Thai man had eaten raw monitor lizard meat and liver. One week later he had a severe headache and 4 days after that he experienced clouding of consciousness. Physical examination revealed no fever, but he was stuporous and had neck stiffness, right hemiparesis, and generalized hyperreflexia. There was no papilledema. A CSF analysis showed high pressure, a white blood cell count of 95 cell/mm3 (eosinophils, 45%; lymphocytes, 33%; neutrophils, 22%), and a CSF protein level of 249 mg/dL. The CSF sugar/blood sugar was 20%. A complete blood count showed leucocytosis with 25% eosinophils.
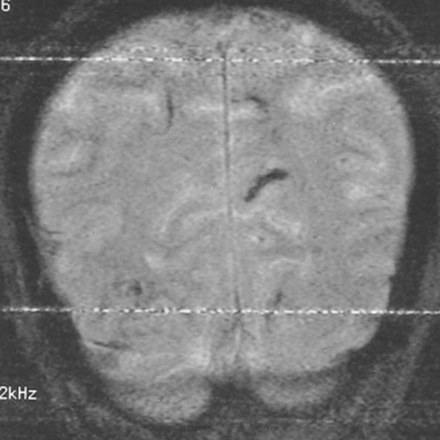
MR examination revealed multiple scattered areas of subcortical enhancing lesions in the frontoparietooccipital regions bilaterally. There was also a tiny enhancing lesion anterior to the fourth ventricle in the right side of the midbrain near the quadrigeminal cistern. Gradient-echo coronal images showed multiple dark signal tracks subcortically, which could have been due to previous hemorrhages (Fig 3). The perivascular subarachnoid space was mildly dilated.
64-year-old man. Coronal gradient-echo MR image (640/25/2) shows linear hypointense subcortical lesions, which might represent hemorrhagic tracks
Case 4
A 28-year-old Thai man was admitted to our hospital 1 week after eating an uncooked snail. He had a severe headache and confusion. Physical examination showed that he was afebrile but drowsy. He had stiffness of his neck, a right facial palsy, and generalized hyperreflexia, but no papilledema or muscle weakness. A lumbar puncture provided confirmatory evidence of eosinophilic meningoencephalitis.
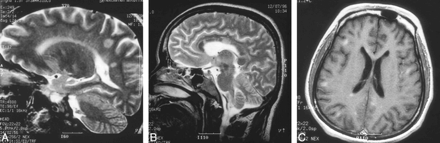
An MR examination revealed round and oval areas of high T2 signal in the periventricular white matter and the corpus callosum (Fig 4A and B). Lesions were also found in subcortical areas, in the callososeptal interface, and in the corona radiata. There were multiple scattered areas of abnormal enhancement, mainly in the subcortical areas and near the posterior horn of the right lateral ventricle (Fig 4C) and in the periaqueductal regions.
A and B, 32-year-old man. Sagittal T2-weighted MR images (4000/98/2) reveal nodular and linear high-signal lesions in periventricular white matter in parietooccipital lobe and corpus callosum.
C, Axial contrast-enhanced T1-weighted image (500/9/2) shows multiple enhancing subcortical lesions and right periventricular lesions.
Case 5
One month after eating raw snails, a 13-year-old Thai boy was admitted to our hospital with a headache and muscle weakness of 2 weeks' duration. He was afebrile and fully conscious, but physical examination showed stiffness of the neck, papilledema, right-sided facial palsy, right lateral rectus palsy, and hemiparesis. CSF analysis revealed high pressure, a white blood cell count of 610 cells/mm3 (eosinophils, 70%; lymphocytes, 30%), and a protein level of 198 mg/dL. His CSF sugar/blood sugar was 50%. A complete blood count showed a leucocytosis with 17% eosinophils.
An MR study revealed tiny subcortical enhancing lesions in the left frontal and right parietal regions and on the superior surface of the cerebellum near the tentorial margin (thought to be due to basal arachnoiditis). An MR spectroscopy voxel placed in the left thalamic region showed normal spectra.
Case 6
A 73-year-old Thai man had eaten raw snails 1 month before admission to our hospital. One week after consuming the snails he experienced a severe headache and became stuporous. On admission, he was afebrile, and physical examination showed papilledema, neck stiffness, and generalized hyperreflexia but no muscle weakness. CSF analysis revealed high pressure, a white blood cell count of 550 cells/mm3 (eosinophils, 50%; lymphocytes, 30%; neutrophils, 20%), and a protein level of 190 mg/dL. His CSF sugar/blood sugar was 30%.
MR studies showed mildly dilated perivascular subarachnoid spaces and areas of increased T2 signal intensity in the periventricular deep white matter, thalamus, centrum semiovale, and body of the corpus callosum. Tiny enhancing lesions in the subcortical left posterior parietal region were noted after injection of contrast material. An MR spectroscopy voxel placed in the right centrum semiovale revealed normal spectra.
The clinical features and MR findings in these six cases are summarized in Tables 1 and 2.
Clinical features of eosinophilic meningoencephalitis
Discussion
A. cantonensis is the most common cause of eosinophilic meningoencephalitis in Thailand. There are several infective agents. P. sp snails when eaten raw are the main source of human infection (7). In the northeastern part of Thailand, the prevalence of infection by P. sp snails has been reported at 0.9% (1). In V. bengalensis (a yellow tree monitor lizard), which can also be a paratenic host for A. cantonensis, the third stage larvae is found mostly in the liver (15, 17). In Chiang Mai province in northern Thailand, a high percentage of the natural definitive rodent hosts are infected with parasites, 42% of them with A. cantonensis (2). In other parts of Thailand, A. cantonensis in rodents is rare (1).
The neuropathology of human angiostrongyliasis has been well described by Rosen et al (5), Jindrak and Alicata (6), Tangchai et al (16), Nye et al (18), and Sonakul (19). In our patients, gross pathologic examination revealed brain congestion and thickened leptomeninges; gross hemorrhage was most unusual. A. cantonensis larvae were easily recognized in the brain tissue, the meninges, and sometimes in the blood vessels or perivascular spaces. In some cases the larvae were densely distributed in the cerebellum and brain stem. At necropsy, the worms were either alive or dead. Cellular reaction was minimal around the living worms but more pronounced around the dead worms. Cellular reactions were also observed along the meninges and intracerebral vessels (16, 18, 19).
One of the most characteristic pathologic features was that of multiple microcavities or numerous tracks (usually smaller than 150 μm) in the brain and spinal cord, representing the passage of migrating worms. Microscopic examination of the tracks showed disruption of brain tissue, debris, gitter cells, and cellular infiltration with or without microscopic hemorrhage. The presence of migratory tracks may be of diagnostic value in some cases. Wallerian or secondary axonal degeneration was present around all of the tracks, probably the result of separation of the axon from the nutrient cell body by the movement of the migrating worm. One striking microscopic feature was vascular dilatation, both arterial and venous, in the subarachnoid spaces. Autopsies of the patients reported by Tangchai et al in 1967 (16) revealed the presence of worms in the subarachnoid spaces over the frontal cortex, pons, tip of the temporal lobes, and paraventricular areas of the lateral ventricle. The subarachnoid space was widened and edematous, and the arachnoid membrane was thickened with marked vascular dilatation in the subarachnoid space.
Radiologic abnormalities in eosinophilic meningitis have been described only rarely. The size and location of lesions varied and were probably related to the number of larvae and their movements. Clouston et al (20) reported three patients in whom eosinophilic meningitis developed after a visit to the Fiji Islands. Two of the patients had severe and long-lasting illness, with chronic intractable pain. In one patient, an MR study performed during the acute phase of the illness showed multiple discrete white matter lesions in both cerebral hemispheres and the cerebellum on T1- and T2-weighted images. In a previous case report of eosinophilic meningoencephalitis caused by A. cantonensis (in a 17-year-old Japanese girl) (21), MR studies of the brain showed multiple small areas of high signal intensity on contrast-enhanced T1-weighted images. MR findings suggested tissue reactions to dead worms and local vasodilatation associated with minimal thrombus formation.
Our MR findings revealed hyperintense signal (cases 1, 2, 4, 5, and 6) due to deeply located lesions. These are nonspecific findings that may indicate demyelination, small vessel ischemic changes, or gliosis. Additional MR spectroscopy was performed in three patients. In one of these (case 1), who was severely ill, choline was decreased and there was white matter damage or axonal degeneration. In case 2, a microcavity with a migratory tract was discovered, possibly surrounded by a variable reaction, degenerated neurons, and associated tissue edema, which were thought to be of diagnostic significance. Abnormal enhancing subcortical, periaqueductal, and basal cisternal lesions (cases 1, 3, 4, 5, and 6) probably related to the eosinophilic inflammatory reaction, causing disruption of the blood-brain barrier. Prominence of the Virchow-Robin spaces (cases 1, 2, 3, 4, and 6), representing dilated subarachnoid spaces and vascular congestion, was another noteworthy feature.
Of the six patients reported here, the three who ate lizard liver had a more severe clinical presentation than did those who ate snails, probably owing to the advanced infectious stage of A. cantonensis (15–17). Furthermore, the patients who ate lizard liver had a tendency to have more intracranial lesions (Table 2), and one patient had a decreased choline spectrum.
Findings at MR imaging and MR spectroscopy
Conclusion
Six male patients presented with eosinophilic meningoencephalitis. Three had consumed raw liver of the monitor lizard (resulting in severe brain damage) while the other three had eaten raw snail. The abnormal MR findings can be classified as periventricular hyperintense T2 signal and enhancing subcortical lesions. Proton MR spectroscopy showed a decreased choline spectrum, which represents white matter damage or axonal degeneration. Microcavity and migratory tracks were also of diagnostic value in one case. MR findings in eosinophilic meningoencephalitis may aid premortem diagnosis and help predict the prognosis by establishing the location of lesions and defining the extent of brain tissue damage.
Acknowledgments
We thank Smarn Tesana for review of the parasitic material.
Footnotes
↵1 Address reprint requests to Jaturat Kanpittaya, MD, Department of Radiology, Faculty of Medicine, Khon Kaen University, Khon Kaen 40002, Thailand.
References
- Received January 14, 1999.
- Accepted after revision December 22, 1999.
- Copyright © American Society of Neuroradiology