Abstract
BACKGROUND AND PURPOSE: Patients presenting with a monosymptomatic episode of neurologic dysfunction (MEND) have a high probability of developing multiple sclerosis (MS). Our study was designed to determine whether magnetization transfer (MT) histogram analysis could predict the development of MS for a cohort of patients presenting with a MEND.
METHODS: Eleven patients with a MEND and 21 age-matched control volunteers underwent MR imaging. Six patients underwent serial MR examinations. MT ratio histogram peak height (MTRHPH) and the location of the MT ratio histogram peak (LOC MTRHP) were determined for patients and control volunteers. T2 lesion volume was also calculated. Patients were clinically followed up for 587 ± 308 days to determine or rule out the development of MS.
RESULTS: Three patients went on to develop MS. There was no statistically significant difference in the MTRHPH (P = .65) and the LOC MTRHP (P = .71) between patients and control volunteers. For those patients who underwent multiple examinations, no statistically significant differences in the MTRHPH (P = .64), LOC MTRHP (P =.58), and T2 lesion volume (P = .47) were seen. There were no statistically significant correlations between any of the parameters studied.
CONCLUSION: We found no difference in MT histogram parameters among control volunteers, patients with a MEND without MS, and patients with a MEND who went on to a diagnosis of MS. Our preliminary findings suggest that there may not be a substrate of disease in the normal-appearing white matter that is predictive of the development of MS.
Based on clinical experience, MR imaging is the best ancillary test to show cerebral abnormalities in patients with clinically definite multiple sclerosis (MS), 95% of whom have lesions revealed by MR imaging (1). This number probably underestimates the sensitivity of MR imaging in that it does not include spinal cord imaging. Combined brain and spinal cord imaging can increase the sensitivity of MR imaging to almost 100% (2). MR imaging criteria for the diagnosis of MS have been proposed based only on conventional proton density–and T2-weighted images (1, 3).
There are many reports indicating that magnetization transfer (MT) imaging is even more sensitive than conventional MR imaging for detecting the presence of MS lesions (4, 5). Abnormal MT ratio (MTR) values have been shown not only in plaques visible on conventional MR images but also in normal-appearing white matter (NAWM) (4, 6–8). The abnormal MTR values in the NAWM have been attributed to microscopic disease (6, 9, 10). MTR values have previously been determined using 2D regions of interest (4–8, 11). This method evaluates the disease status of tissues locally, because it is determined from the voxel values within these regions. Although this is a satisfactory method for the detection of local disease processes or the interrogation of individual lesions, it cannot quantitate global disease burden in a diffuse or multifocal disease such as MS.
The purpose of this study was to determine whether patients presenting with a monosymptomatic episode of neurologic dysfunction (MEND) would have both macroscopic and microscopic disease that could be detected by MTR histogram analysis. Our hypothesis was that, using MTR histogram analysis, we would be able to detect disease in the brains of patients with a MEND who eventually went on to a diagnosis of MS (9, 10, 12). Our aim was also to differentiate those patients with a MEND who went on to a diagnosis of MS as defined by the Poser criteria from those patients with a MEND who did not develop MS (13) by using the MTR histogram peak height (MTRHPH). Although several studies using MTR histogram analysis have been reported in the literature (9, 10), to our knowledge, this is the first report of an MTR histogram analysis of patients with a MEND.
Additionally, we investigated whether MT would be more prognostic than T2 lesion volume for predicting the development of MS in the same cohort of patients presenting with a MEND, considering that MT is considered to be an extremely sensitive technique for quantitation of global disease burden.
Methods
Our patient cohort consisted of 11 patients (seven women and four men; age range, 23–51 years; mean age, 35.1 years) with a MEND. All patients were recruited from the Comprehensive Multiple Sclerosis Center at our institution and were under the care of a neurologist specializing in MS. An age-matched control group of 21 healthy volunteers (10 women and 11 men; age range, 28–61 years; mean age, 38.9 years) also underwent examination. Those with a medical history of systemic or neurologic disease were excluded from the study. A second cohort, consisting of six of the 11 patients from the first cohort, were followed longitudinally by serial MR and clinical examinations. Three patients underwent three MR examinations each, and three patients underwent two examinations each. In these patients, the interval between the first and last MT and T2-weighted studies varied from 169 days to 906 days (mean time, 292 days ± 106 days). All patients were followed up clinically. Written informed consent was obtained from each participant entering the study. Additionally, the institutional review board approved the study.
All MR examinations (17 for patients and 21 for control volunteers) were performed on the same 1.5-T system (Signa; GE Medical Systems, Milwaukee, WI) with a quadrature transmitter/receiver head coil. After obtaining sagittal localizer T1-weighted spin-echo images of the whole brain (600/11/1 [TR/TE/excitations]), 3-mm contiguous axial fast spin-echo images of the whole brain (2500/16−80/1) were acquired with a 256 × 192 matrix and a 22-cm field of view. Using MT parameters previously established by other investigators, unenhanced axial MT images were obtained (4, 5). A standard 3D gradient-pulse sequence (106/5/1, flip angle of 12°) with a 256 × 128 matrix and a 22-cm field of view was used. MT images were obtained by the application of 19-ms single-cycle, sinc-shaped saturation pulses before each excitation. The RF pulses had an average field intensity of 3.67 × 10−6 T (156 Hz) and were applied at a frequency of 2 kHz below the resonance of water (14). The interval between the end of the saturation pulse and the beginning of each excitation was approximately 1 ms. Five-millimeter-thick axial images at similar intervals were also obtained without the saturation pulses but with otherwise identical imaging parameters.
All image data were transferred from the imager directly to a Sun Sparc 20 (Sun Microsystems, Mountain View, CA) workstation (four processors, 256 MB RAM) via the picture archiving and communications system of the radiology department. An internal version of the 3DVIEWNIX software system, which has been previously described by Udupa et al (15, 16) and Samarasekera et al (17), was used to measure T2 lesion volume. The operator initially defined CSF, white matter, and gray matter using long recovery time/short echo time as well as long recovery time/long echo time MR images. The software automatically then selected and delineated potential lesion sites, using a method based on a theory of “fuzzy connectedness” (18). The operator subsequently determined which were the “true” lesions among the computer-detected potential lesions. The T2 volume of the lesions was then computed by summation of the volume of the lesions accepted by the operator. Our algorithm used to quantitate T2 volumes has been reported to have an intraobserver and interobserver coefficient of variation of 0.9% and a false volume fraction of 1.3% (15). All operator input needed for T2 volume estimation was provided by the same person (J.S.K.), who was blinded to whether the patients had developed MS by the time of clinical follow-up.
The volume of CSF and brain parenchyma were also assessed using 3DVIEWNIX software. After an operator specified sample points of CSF, white matter, and gray matter, the extracranial contents were segmented. CSF, white matter, and gray matter were then segmented, with each being treated as a fuzzy-connected 3D object containing the above-specified points (16). An operator then reviewed all the segmented sections, and any remaining extracranial contents were excluded.
The amount of MT was quantitated by calculating the MTR. The MTR, defined as the percentage of signal loss between otherwise identical images obtained with and without saturation, was calculated by using the following equation (4): 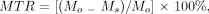 where Mo and Ms represent the signal intensity of a voxel in the image obtained for the same patient and for the same acquisition without and with saturation, respectively.
where Mo and Ms represent the signal intensity of a voxel in the image obtained for the same patient and for the same acquisition without and with saturation, respectively.
The 3DVIEWNIX software system was then used to segment the whole brain parenchyma from the MT images and to generate a histogram of the pixel intensity from the MTR maps of only the whole brain. To compare the MTRHPHs of patients and control volunteers with different brain volumes, the MTRHPHs were normalized by dividing the peak height of the MTR histogram by the total number of voxels in the brain parenchyma. In the subsequent discussion, we refer to the normalized whole-brain MTRHPH as MTRHPH. The algorithms used for generating the whole-brain MTR histograms have been previously described in detail by van Buchem et al (9).
The MTR histogram parameters of patients and control volunteers were compared using a Wilcoxon ranked sum test for independent samples; P values of less than .05 were considered to represent a significant difference in results between patients with a MEND and control volunteers. Both the patient and control groups were evaluated retrospectively to determine whether the groups were matched in terms of age.
The MTRHPH (proportion of pixels at the most common MTR value) reflects the amount of residual normal brain parenchyma (9, 10) in that it typically has a narrow peak located at an MTR value of 40% in both patients with MS and normal control volunteers. The narrow peak indicates that most of the brain tissue contains MTR values within a small range. Although the MTRHPH in patients with MS is centered near the MTR of normal control volunteers, the average height of the peak in patients with MS is significantly lower because of a relative increase of the number of pixels with lower MTR values that correspond to plaques and NAWM in patients with MS (4, 10). Considering our assumption that patients with a MEND would be similar to patients with MS, the MTRHPH and the location of the whole-brain MTR histogram peak (LOC MTRHP) were determined for both patients and control volunteers.
T2 lesion volumes were also obtained for the patients. Additionally, for the longitudinal part of the study involving the six patients who underwent multiple MR examinations, the percentage change per unit time of the MTRHPH, LOC MTRHP, and T2 lesion volume was also made between the first and the last MR examinations. We also examined whether there was a correlation between the MTRHPH, LOC MTRHP, and T2 lesion volume for both the patients and the control volunteers by using Spearman correlation coefficients.
Results
Table 1 lists the patient examinations along with the details pertaining to symptoms, acquisition times, and the disease parameters computed from the images. There was no statistically significant difference in age between the patients and the control volunteers. The mean value of age was 35.1 ± 8.8 years (mean ± SD) for the 11 patients with a MEND and was 38.90 ± 10.0 years for the control volunteers. There was also no statistically significant difference in the MTRHPH (P = .65) and the LOC MTRHP (P = .71) between the patients and the control volunteers (Table 2). Although limited by a small sample size, our preliminary results show that for those patients who underwent multiple MR examinations, no statistically significant differences were seen over time in the MTRHPH (P = 1.00), LOC MTRHP (P = .81), and T2 lesion volume (P = .44).
MEND patients symptoms and data
Results comparing MTRHP and LOC MTRHP between patients and control subjects*
There were no statistically significant differences between patients and control volunteers regarding any of the parameters (MTRHPH, LOC MTRHP, and T2 lesion volume) at the time of the initial MR examination (Table 3) or at the subsequent MR examinations of those patients entered in the longitudinal part of the study.
Results of correlations among the parameters used to evaluate patients and control subjects*
Three of the 11 patients went on to a diagnosis of MS as defined by the Poser criteria (13). A statistical analysis comparing patients with a MEND who went on to develop MS and patients with a MEND who did not go on to develop MS was not performed. There would not have been enough statistical power for a meaningful result considering that only a few patients in our study went on to develop MS. No obvious differences were seen, however.
Discussion
Serial MR imaging may aid in the diagnosis of MS, particularly for a patient suffering from a MEND. New lesions identified on MR images more than 1 month after the initial clinical presentation indicate clinically probable MS according to the Poser criteria (13). More recently, Barkhof et al (19) suggested that the presence of juxtacortical lesions and contrast-enhanced lesions among patients with monosymptomatic neurologic dysfunction are highly specific prognostic factors for progression to MS. Those patients presenting with isolated acute syndromes and normal MR images of the brain are at a lower risk of progressing to MS (20, 21). The presence and number of lesions on MR images of the brain markedly increase the risk of progression to MS, not only in cases presenting with optic neuritis but also in cases of isolated spinal cord and brain stem syndromes (20–26). In the Optic Neuritis Treatment Trial, only 16% of patients with optic neuritis and normal MR images went on to develop MS as compared with 51% of patients with optic neuritis and abnormal MR images at symptom onset (21). Moreover, Miller et al (27) found that progression to MS occurred in 42% of patients with isolated spinal cord dysfunction and in 57% of patients with isolated brain stem manifestations. Thus, MR imaging serves as a powerful predictive tool for determining the risk of MS among patients presenting with a MEND (20, 22).
Our sample size may be too small from which to reach any definitive conclusions; a larger number of patients may be necessary to verify our hypothesis because of the possible subtle changes. Nevertheless, our preliminary results show no statistically significant difference in MT histogram parameters (ie, the MTRHPH and the LOC MTRHP) among control volunteers, patients with a MEND without MS, and patients with a MEND who developed MS. These findings suggest that there may not be an initial substrate of diffuse disease in individuals with a MEND; specifically, our patient cohort with monosymptomatic disease does not seem to have a diffuse low-level lesion base on MT histograms. Moreover, the initial disease burden as assessed by the initial T2 lesion volume did not correlate with the development of MS. Additionally, no significant correlations were seen between the development of MS and the three parameters (MTRHP, location of MTRHP, and T2 lesion volume) evaluated.
Our preliminary results of the three of 11 patients with a MEND whose conditions evolved to MS show that no predictive features could be detected by our methodology. Possible limitations of our study include the notion that whole-brain MT imaging using MTR histogram analysis may not be sensitive enough for detecting diffuse subtle changes in the NAWM. Alternatively, minimal microscopic disease may be focal and overwhelmed by the whole-brain MTR histogram approach. Other methodologies, including proton spectroscopy, may provide increased sensitivity in this important cohort of patients.
The mean interval between the first and last MR examinations of patients who underwent multiple MR examinations was 292 ± 106 days; this may have been too short a period during which to detect progressive changes in patients with a MEND. van Buchem et al (10) found a decrease in MTRHP in their cohort of seven patients studied longitudinally, and they proposed that the MTRHP could be used as a volumetric tool that is sensitive to differences in global lesion load occurring in a relatively short period of time. These patients, however, were studied over a longer period of 25 months (approximately 760 days). Moreover, in a study by Lacomis et al (28), T1 times were prolonged in NAWM for patients with clinically diagnosed MS of at least 5 years' duration. Nevertheless, this finding was not observed among patients with MS of less than 5 years' duration. Because all the patients in our study were followed for less than 5 years, changes in the NAWM may not have been apparent during the shorter interval of this study.
The mean time between the first MR examination and the final clinical follow-up for patients who did not develop MS was 692 ± 244 days (or 587 ± 308 days if patients who developed MS are included). This interval may be too short a period to detect which patients will develop MS. In a study by Filippi et al (22), more than three-quarters of their patients with a MEND who developed MS did so during the first 2 years. Likewise, Cohen et al (29) showed that the risk of developing MS for patients with isolated optic neuritis is higher during the first 2 years after onset.
Conclusion
In cases of MS, a disease in which clinical signs and symptoms correspond poorly with the extent and activity of disease, quantitative data reflecting the burden of disease would be useful in understanding the natural course of the disease and in the assessment of therapeutic intervention. Our data indicate that using MR imaging data, such as whole-brain MT imaging and T2 lesion volume, it may be difficult to predict which patients with a MEND will go on to develop MS. This suggests that MR imaging criteria for entry into therapeutic trials, before a diagnosis of definite MS has been established, may result in incorrect characterization and perhaps unnecessary treatment.
Acknowledgments
We especially thank Lois Mannon for help in recruiting patients for the study.
Footnotes
↵1 Address reprint requests to Robert I. Grossman, MD, Neuroradiology Section, Department of Radiology, University of Pennsylvania Medical Center, 3400 Spruce Street, Philadelphia PA 19104.
2 Presented at Annual Meeting of the Radiological Society of North America; November 1998; Chicago, IL.
3 Funded in part by the National Institute of Neurological Disorders and Stroke, National Institutes of Health Grants NS37172 and NS29029.
References
- Received June 1, 1999.
- Accepted after revision January 10, 2000.
- Copyright © American Society of Neuroradiology











